Pro-inflammatory cytokines levels in tears and dry eye disease in Parkinson’s disease
Highlight box
Key findings
• Parkinson’s disease (PD) patients have higher tumor necrosis factor-alpha (TNF-α) levels in their tears when compared to controls.
What is known and what is new?
• Neuroinflammation plays a critical role in PD, and pro-inflammatory cytokines are key players in the immune response.
• Patients with PD exhibit elevated levels of TNF-α in tears, suggesting the potential involvement of this cytokine in neuroinflammation in PD.
What is the implication, and what should change now?
• The elevated TNF-α levels in tears of PD patients suggest that it could serve as a potential biomarker for neuroinflammation in PD.
• Monitoring tear cytokine levels could aid in early diagnosis and monitoring therapeutic strategies for PD.
Introduction
Parkinson’s disease (PD) affects around 1% of individuals above 60 years of age; it is the second most common degenerative disorder (1). Bradykinesia, tremor, and rigidity are the primary motor symptoms in these patients. The prodromal phase is characterized by non-motor symptoms such as olfactory dysfunction, psychiatric symptoms, autonomic system dysfunction, sleep disorders, pain, and fatigue (2). The pathogenesis of PD has not been clarified in detail. Evidence supports the link of PD with chronic neuroinflammation and microglia activation. Increased levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-12p70, and interferon-gamma (IFN-γ) can stimulate the recruitment of peripheral leukocytes to the central nervous system. A permanent pro-inflammatory state results in chronic inflammation (3-5).
Moreover, high levels of specific cytokines have been linked to the severity of motor and non-motor symptoms such as depression, fatigue, and cognitive impairment in PD (6-10). Increased levels of cytokines IL-1β, IL-2, IL-4, and IL-6 in cerebrospinal fluid (11,12) and in the striatum of the postmortem brain of PD patients have been reported (13).
PD patients have been reported to present a higher prevalence of ophthalmologic conditions such as dry eye disease (DED) than control patients (60–86% vs. 5–30% ) (14,15). DED in PD has been explained by a decreased blink rate (BR) and subsequent reduction of the lipid layer over the cornea, which leads to the aqueous layer evaporating. Moreover, reduced tear production due to autonomic dysfunction has been reported (16,17). Pathogenesis of DED is associated with ocular surface inflammation. Several pro-inflammatory cytokines have been found elevated in tears from DED subjects (18).
TNF-α is considered a major pro-inflammatory cytokine in neuroinflammation. TNF-α and other cytokines are released for neuroprotection, but in later stages, they become neurotoxic (19). In addition, in one study of postmortem PD brains, the areas with significant loss of dopaminergic neurons were those with the highest levels of TNF-α (20-22). Interestingly, elevated levels of TNF-α have been found in the tears of PD patients compared with healthy controls (HC) (23). These increased levels of cytokines in the tears of PD patients may be explained by the underlying pathophysiology, bradykinesia, and DED. The current study was designed to quantified the levels of cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α) in tears in PD and HC, and to evaluate whether these inflammatory cytokines correlated with disease clinical characteristics and DED indicators. We hypothesized that PD patients will have higher levels of cytokines in tears in comparison to HC and that these levels will correlate with motor severity and DED markers. To the best of our knowledge, this is the first study to investigate the quantification of the pro-inflammatory profile in tears and also the existence of DED in PD patients. DED is usually linked to an inflammatory profile and is seen frequently in patients with PD. We present this article in accordance with the STROBE reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-22-70/rc).
Methods
Subjects and controls
Sixteen subjects with idiopathic PD and 16 age- and sex-matched HC were included. They attended the Movement Disorder Outpatient Clinic at the National Institute of Neurology and Neurosurgery in Mexico City and were enrolled. HC were volunteers recruited from an ophthalmological hospital. Written consent was obtained from all participants. This study was performed following the ethical standards of the Helsinki declaration (as revised in 2013) and was approved by the institutional review board and local ethics committee of the National Institute of Neurology and Neurosurgery, Mexico City, Mexico (No. 162-19).
All subjects underwent medical history evaluation and physical, neurological, and ophthalmological examinations. Patients were assessed according to the United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnosis Criteria to confirm the idiopathic PD diagnosis. We included demographic information like age, age at disease onset, PD duration, and predominant side of symptoms. We recorded Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (24), this is a comprehensive assessment tool designed to evaluate various motor and non-motor aspects of PD. It consists of four parts that assess different domains of the disease. Part I—non-motor experiences of daily living: this part assesses the impact of non-motor symptoms on daily activities and quality of life. It includes questions related to mood, cognition, hallucinations, apathy, sleep disturbances, and other non-motor aspects. The scale evaluates the frequency and severity of these symptoms and their impact on the patient’s functioning. Part II—motor experiences of daily living: this section focuses on motor symptoms and their impact on activities of daily living. It covers areas such as speech, salivation, swallowing, handwriting, cutting food, dressing, hygiene, falling, and freezing of gait. The scale rates the severity and frequency of these motor symptoms and their impact on the patient’s ability to perform daily tasks. Part III—motor examination: part III is a comprehensive assessment of motor symptoms using a standardized examination protocol. It evaluates various aspects of motor function, including tremor, rigidity, bradykinesia (slowness of movement), and postural instability. The examination assesses motor symptoms at rest, during posture maintenance, and during various movements. It provides a detailed evaluation of motor impairment and helps track disease progression. Part IV—motor complications: this part focuses on motor complications that may arise as a result of long-term treatment with dopaminergic medications. It assesses motor fluctuations (changes between “on” and “off” states) and dyskinesias (abnormal involuntary movements). The scale evaluates the frequency, severity, and impact of these complications on daily activities and quality of life. Each part of the MDS-UPDRS is scored independently, and the scores from all four parts are often combined to obtain an overall assessment of disease severity and progression. The scale provides a standardized and comprehensive evaluation of both motor and non-motor symptoms, helping clinicians and researchers assess the impact of PD and track changes over time.
Montreal Cognitive Assessment (MoCA) (25), and disease stage were evaluated by the Hoehn and Yahr (HY) stage scale (26). Scales were assessed by a movement disorder specialist. The permission for the use of scales was requested and obtained according to the instructions for members of the International Parkinson Movement Disorders Society. Postural instability and gait disturbance (PIGD) motor subtype, hallmarked by bradykinesia and axial symptoms, and PIGD score were determined according to Stebbins et al. (27).
Exclusion criteria for controls were subjects with familial history of PD, parkinsonism, tremor, dementia by Lewy bodies, Alzheimer’s disease and/or any other alpha synucleinopathy, systemic diseases including inflammatory, autoimmune, hematologic, neoplasia disease, diabetes mellitus, thyroid disease, alcohol addiction, acute or chronic infection, hepatic of renal failure, use of diuretic, anti-inflammatory, antineoplastic, corticosteroid, immunosuppressive, antidepressant or anxiolytic drugs within the last 2 months; and use of topical drops within the last month. The control group was excluded if the systemic or ocular medication caused possible eye problems.
In addition, subjects with an active ocular infection or signs of inflammation, ocular allergy, pterygium, use of ophthalmic drops, use of contact lenses, or ocular surgery in the last 3 months were also excluded. Only the right eyes of PD and HC subjects were used for analysis.
Dry eye evaluation included the following
Tear break-up time (TBUT) and fluorescein staining
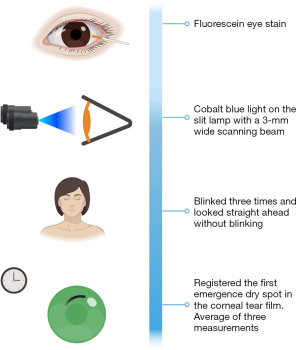
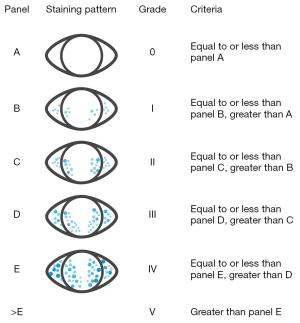
A sterile Ful-Glo® Fluorescein Sodium Ophthalmic Strip (Sola/Barnes-Hind, Phoenix, AZ, USA) wetted with a drop of non-preserved buffered saline was placed into the external third of the lower eyelid. The TBUT measurement was then taken using the cobalt blue illumination on the slit lamp with a 3-mm-wide scanning beam in each eye. After the instillation of fluorescein, the participant was asked to blink three times and, after that, to look straight ahead without blinking. The time interval between a complete blink and the first emergence of a dry spot in the corneal tear film was measured. The average of three successive measurements of the TBUT test was calculated (Figure 1). The result was considered positive if TBUT was less than 10 seconds (28). Corneal staining was graded using the Oxford scheme (Figure 2). An increased staining panel labeled A to E according to the severity is used (A, less severe, E, more severe). Staining ranges from 0 to V for each panel and 0 to 15 for the entire exposed inter-palpebral area. The staining grade is represented by punctate dots, increasing by 0.5 of the logarithms of the number of dots between panels B and E (29).
BR
In the absence of a provoking external stimulus, a bilateral paroxysmal closure of the eyelids lasting less than one second was considered to be a blink. In a 3-minute video recorded while the patient was having a conversation, the “BR” was defined as the average rate at which the eyelids were closed per minute. Blinks were considered complete if at least half of them involved rapid eye reopening and eye closure (30,31).
Dry Eye Questionnaire 5 (DEQ-5)
The DEQ-5 (32) focuses on three common DED symptoms: eye discomfort, eye dryness, and watery eyes. Scores of ≥6 indicate mild dry eye symptoms, and scores of ≥12 indicate severe symptoms. For study purposes, DED was defined as positive symptoms measured by DEQ-5 and a decreased TBUT (<10 seconds) or ocular surface staining (Oxford schema ≥ grade III) (33).
Tear collection sample and flow cytometry analysis
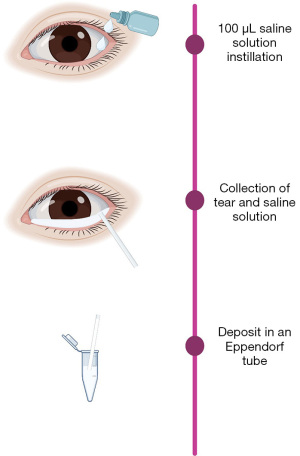
Unstimulated 100 µL tear samples were collected non-traumatically from the outer canthus of open eyes using capillary tubes during the collection sample. Tear samples were stored at −80 ℃ until assayed (Figure 3). BD Cytometric Bead Array Human Inflammatory Cytokine Kit (BD Biosciences, USA) was used to assess the cytokine profile (allowing one to measure the concentration of IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α) in tear samples.
The tests were performed according to the manufacturer’s protocols (in this link you can find the information on the cytokine kit: https://www.bdbiosciences.com/en-us/products/reagents/immunoassay-reagents/cba/cba-kits/human-inflammatory-cytokine-cytometric-bead-array-cba-i-kit.551811). BD Cytometric Bead Array Human Inflammatory Cytokines Kit: 50 µL of assay beads and 50 µL of the studied sample was added consecutively to each sample tube. The samples were incubated at room temperature in the dark for 1.5 hours. Next, the samples were washed with 1 mL of Wash Buffer, centrifuged, and the resulting pellet was resuspended in 50 µL of detection reagent. The samples were further incubated for 1.5 hours, washed, and centrifuged. After discarding the supernatant, the pellet was resuspended in 300 µL of wash buffer and analyzed on the same day in a flow cytometer. Before the analysis, the cytometer was calibrated using set-up beads according to the manufacturer’s protocol.
Statistical analysis
All statistical data analyses were performed with SPSS 25.0 Statistics Inc. and graphs were created with GraphPad Prism 9. The Shapiro-Wilk test was used to examine if the continuous variables were normally distributed. Demographic and clinical data were calculated with mean values and standard deviation for continuous variables. Wilcoxon matched pair signed rank (two samples) was used to compare cytokine concentrations between PD subjects and controls. Mann-Whitney U test was done to compare PD clinical characteristics between DED and subjects without DED. Correlation between cytokines concentrations and age, age on onset, PD duration, MDS-UPDRS I–IV, TBUT, BR, and DEQ-5 was assessed using Spearman’s rank correlation coefficient. Statistical differences were considered significant if P<0.05.
Results
A total of 16 subjects with PD and 16 age- and gender-matched HC were included. The mean age of all PD subjects was 63.25±12.73 years. Regarding gender, 68.8% (n=11) were male and 31.3% (n=5) were female. The side of the initial onset of the disease was right at 56.3% (n=9). The demographic and clinical characteristics of PD subjects and HC divided by a group with DED and without are shown in Table 1.
Table 1
| Clinical characteristics | PD subjects with DED (n=8) | PD subjects without DED (n=8) | HC with DED (n=2) | HC without DED (n=14) |
|---|---|---|---|---|
| Sex, male (%) | 66.7 | 71.4 | 50.0 | 71.4 |
| Age (years) | 64.25±14.08 | 62.25±12.10 | 80±9.89 | 60.85±11.40 |
| Age of PD onset (years) | 52.28±11.25 | 54.37±14.09 | NA | NA |
| Duration of disease (years) | 14.57±12.51 | 7.87±3.18 | NA | NA |
| MDS-UPDRS I score | 13.25±7.68 | 10.25±3.84 | NA | NA |
| MDS-UPDRS II score | 16.87±13.33 | 7.0±3.07 | NA | NA |
| MDS-UPDRS III score | 41.12±20.0 | 19.62±13.99 | NA | NA |
| MDS-UPDRS IV score | 4.0±4.98 | 0 | NA | NA |
| MDS-UPDRS total score | 62±35.79 | 26.62±16.48 | NA | NA |
| HY stage | 2.62±1.30 | 1.5±0.53 | NA | NA |
Data are presented as mean ± SD. PD, Parkinson’s disease; HC, healthy control; DED, dry eye disease; MDS-UPDRS, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale; HY, Hoehn and Yahr; NA, not applicable.
Frequency of DED
According to DEQ-5, 50.0% (n=8) of PD subjects and 12.5% (n=2) controls had DED symptoms. In PD group, mild symptoms were present in 37.5% (n=6) and severe symptoms in 12.5% (n=2). The pro-inflammatory cytokine profile of HC with DED was: IL-1 (0.11±0.16 pg/mL), IL-6 (2.10±2.14 pg/mL), IL-8 (92.79±27.45 pg/mL), IL-10 (0 pg/mL), IL-12p70 (0.41±0.57 pg/mL), and TNF-α (0.1±0.01 pg/mL).
Statistical differences were found in TBUT, BR, and DEQ-5 when comparing PD subjects with PD. The mean of TBUT was lower in PD when compared to HC (8.18±4.03 vs. 13.5±3.74 seconds, P=0.001). TBUT was less than 10 seconds in 62.5% (n=10) of PD subjects and 12.5% (n=2) of controls. The mean DEQ-5 score was higher in PD than in HC (5.68±4.39 vs. 2.18±1.64, P=0.002). The mean BR was 10.37±2.68 blinks/minute in PD and 16.31±4.45 blinks/minute in HC (P=0.001).
The corneal fluorescein staining by Oxford schema the 25.0% (n=4) had a grade 0, 31.3% (n=5) had a grade I, 6.3% (n=1) had a grade II, 25.0% (n=4) had a grade III, and 12.5% (n=2) had a grade IV in PD group. In contrast to the control group, 43.8% (n=7) had a grade 0, 37.5% (n=6) had a grade I, 12.5% (n=2) had a grade II, and 6.3% (n=1) had a grade III.
A higher BR correlated moderately with a lower HY stage (r=−0.645, P=0.007).
Eight PD patients and two controls fulfilled the study’s operational definition of DED.
Comparison of cytokine profile between PD subjects and controls
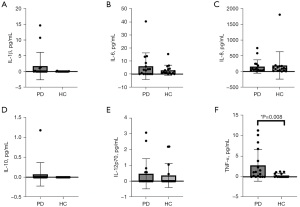
The TNF-α concentration in tears was higher in PD subjects than controls (2.94±3.95 vs. 0.33±0.49 pg/mL, P=0.008). There was no difference between IL-1β, IL-6, IL-8, IL-10, and IL-12p70 concentration in tears of PD subjects vs. controls. These results are shown in Figure 4.
There was no evidence for a relationship between IL-1β, IL-10, and TNF-α tear concentration and any of the clinical characteristics assessed in PD subjects. In contrast, IL-6 positively correlated with duration symptoms (r=0.575, P=0.025). While tear concentration of IL-8 was correlated with age (r=0.545, P=0.029), HY (r=0.532, P=0.034), MoCA (r=−0.511, P=0.043), TBUT (r=−0.547, P=0.028), and DEQ-5 (r=0.605, P=0.013).
Comparison between PD subjects with and without DED
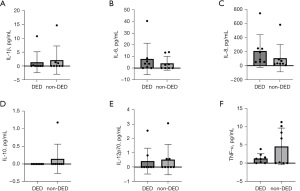
PD subjects with DED had a slower BR than PD without DED (8.87±2.16 vs. 11.87±2.47 blinks/minute, P=0.038). Regarding motor evaluation, PD subjects had the worst MDS-UPDRS III score (41.12±20.0 vs. 19.62±13.99, P=0.007). Nevertheless, there was no difference in the PIGD score between groups (1.75±1.13 vs.1.31±1.90, P=0.195) nor in the frequency of PIGD subtype (P=0.315). No differences were found in cytokine concentrations in tears between PD patients with DED compared to those without DED. These comparisons are presented in Table 2 and Figure 5.
Table 2
| Clinical characteristics and cytokines | DED (n=8) | Non-DED (n=8) | P value |
|---|---|---|---|
| Age (years) | 64.25±14.08 | 62.25±12.10 | 0.721 |
| Years of PD | 14.57±12.51 | 7.87±3.18 | 0.463 |
| MDS-UPDRS I score | 13.25±7.68 | 10.25±3.84 | 0.442 |
| MDS-UPDRS II score | 16.87±13.33 | 7.0±3.07 | 0.015* |
| MDS-UPDRS III score | 41.12±20.0 | 19.62±13.99 | 0.007* |
| MDS-UPDRS IV score | 4.0±4.98 | 0 | NA |
| MDS-UPDRS total score | 62±35.79 | 26.62±16.48 | 0.005* |
| HY stage | 2.62±1.30 | 1.5±0.53 | 0.065 |
| PIGD score | 1.75±1.13 | 1.31±1.90 | 0.195 |
| IL-1β (pg/mL) | 1.37±3.78 | 2.13±5.10 | 0.645 |
| IL-6 (pg/mL) | 7.88±13.49 | 4.14±5.93 | 0.442 |
| IL-8 (pg/mL) | 209.23±232.65 | 107.16±194.06 | 0.065 |
| IL-10 (pg/mL) | 0 | 0.14±0.41 | NA |
| IL-12p70 (pg/mL) | 0.42±0.90 | 0.52±1.05 | 0.798 |
| TNF-α (pg/mL) | 1.28±1.32 | 4.61±5.04 | 0.645 |
| Blink rate (blinks/minute) | 8.87±2.16 | 11.87±2.47 | 0.038* |
Data are presented as mean ± SD. *, P was considered significant when <0.05. PD, Parkinson’s disease; DED, dry eye disease; MDS-UPDRS, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale; HY, Hoehn and Yahr; IL, interleukin; PIGD, Postural Instability and Gait Disturbance; TNF-α, tumor necrosis factor-alpha; NA, not applicable.
Discussion
Biomarkers are surrogate indicators of both standard and pathogenic biological processes. They are helpful for an early and accurate diagnosis, predicting the severity of the disease, and identifying effective therapeutic interventions (34). In PD identifying new biomarkers has become increasingly helpful over the past decades. These can help to detect patients at risk and may allow for an early diagnosis and management (35). The lacrimal tear is a biological fluid that can easily be collected and is considered a more accessible, less invasive, and less complex biological fluid than other tissues (36,37).
Cytokines are immunological messengers that contribute to neuroinflammatory cascades in PD. Several studies showed increased cytokines levels in PD subjects compared with controls in serum, cerebrospinal fluid and tears. Pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α are produced by microglia in the substantia nigra (SN); also, anti-inflammatory cytokine-like IL-10 is present in PD subjects (38-40). It is reported that these cytokines are linked to microglial activation as a consequence of excessive localized brain inflammation and neurotoxicity in neurodegenerative diseases (41).
IL-1β is a pro-inflammatory cytokine implicated as the main effector of the functional consequences of neuroinflammation on neurodegeneration in PD models. Prolonged pro-inflammatory IL-1β expression in the SN results in significant and permanent dopaminergic neuronal death in the SN (42).
IL-8 is a chemokine produced by macrophages. Its receptor, the CXCR2 has been detected in dystrophic neurites, suggesting that IL-8 mediates glial interactions with neurons and thereby contributes to neuronal damage (43). Previous studies have shown inconsistent results of serum IL-8 levels in PD. Williams-Gray et al. (44) and Fu et al. (45) reported no statistical difference, but Gupta et al. (46) showed that IL-8 was significantly decreased in the serum in PD compared to controls (patients =222.70±200.91 pg/mL, controls =1,584.44±504.44 pg/mL; P<0.001). Also, serum IL-8 level was positively associated with disease duration, depression, and UPDRS III in PD patients (46,47).
IL-10 is an anti-inflammatory cytokine modulator of glial activation in neurodegenerative diseases. High levels have been reported in PD compared with HC (48). However, in another study, it was described that PD patients with more severe clinical characteristics and a non-tremor type showed reduced serum IL-10 levels (49).
TNF-α is a pro-inflammatory cytokine strongly correlated with PD pathology. In pathological conditions, astrocytes and microglia release large amounts of TNF-α. This process could contribute to the neuroinflammation seen in PD (21,50).
On the other hand, PD patients can concomitantly present DED, which is accompanied by a similar cytokines profile. The study’s objective was first to determine the levels of cytokines in tears and compare them with age- and sex-matched controls and second, to correlate those findings with the motor symptoms and DED in PD. In our study, analogous to the literature, TNF-α tear levels were notably higher in PD subjects than in controls. Nevertheless, these levels were not linked to age, gender, age at onset, PD duration, motor symptoms, cognitive symptoms, or HY stages (23).
As expected, DED was more frequent in PD subjects than in control subjects, similar to what was reported in the literature, estimated at 60–86% in PD (14). Also, PD subjects had poorer TBUT and higher DEQ-5 scores than controls. Söğütlü Sarı et al. (30) found decreased levels of TBUT in 37 PD subjects than in controls (11.3 vs. 12.8 seconds), but the difference was not significant. Similarly, Reddy et al. (16) described no significant difference in TBUT (8.4±1.9 vs. 9.8±0.4 seconds) in PD patients. However, Biousse et al. (51) reported that BR and TBUT were decreased in 30 PD patients compared with controls in untreated early-onset PD subjects.
In PD incomplete blinking and a decrease in BR can be observed due to a deficiency in dopamine. These changes could lead to tear film hyperosmolality, accelerated tear evaporation, and finally corneal damage causing DED (52). In our study, a higher BR correlated moderately with a lower HY stage (r=−0.645, P=0.007), and patients with DED have lower BRs (12.14±2.54 vs. 9.0±2.06, P=0.031). In contrast, Fitzpatrick et al. could not find an association between BR and disease severity (53).
In addition, increased inflammatory cytokines have been detected in the tear film and conjunctival epithelium produced by the conjunctival and lacrimal gland, in accordance with the chronic inflammatory process in DED (54-57). Massingale et al. (18) analyzed cytokines in tear samples of seven subjects with DED and seven normal controls. They reported that all cytokines were increased in the tears of DED subjects compared with controls. In our study, IL-1β, IL-6 and IL-10 tear levels were higher in PD subjects than in controls, but this difference was not statistically significant probably due to a small sample size. Also, there was no evidence for a relationship between IL-1β, IL-6, IL-10, IL-12p70 and TNF-α tear concentration and clinical characteristics in PD subjects. Our study described that IL-8 was decreased in PD and presented association with the HY stage (r=0.523, P=0.034).
Interestingly, this study showed significant differences among patients with DED in PD regarding their BR and HY stage. These findings are consistent with the hypothesis that PD patients developed DED due to lower BY and subsequently increased pro-inflammatory cytokines.
Our study has several limitations, the sample size was too low, and further studies with larger numbers of patients were needed to confirm our findings. In addition, we used the Human Inflammatory Cytokine Kit to evaluate tear cytokines, while other reports in the literature used multiplex immunobead assay. Although we did not measure IL-4 and IFN-γ, it has been reported they are increased in serum of PD patients and linked to neuroinflammation. Moreover, a comparison between cytokines levels in serum and tears was not carried out. As mentioned before, our patient samples did not reflect the different stages of the disease.
Conclusions
PD patients have higher levels of TNF-α tears than age- and sex-matched HC. IL-8 in tears may be involved in the disease severity and DED in PD. Also relevant, the mechanisms behind the DED in PD seem to be related to BR and the severity of the disease. These findings should be confirmed in a larger group of patients.
Acknowledgments
Figures 1-3 were created with BioRender.com.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-22-70/rc
Data Sharing Statement: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-70/dss
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-70/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-70/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board and local ethics Committee of the National Institute of Neurology and Neurosurgery, Mexico City, Mexico (No. 162-19). Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev 2014;14:19-30. [Crossref] [PubMed]
- Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896-912. [Crossref] [PubMed]
- Ferrari CC, Pott Godoy MC, Tarelli R, et al. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis 2006;24:183-93. [Crossref] [PubMed]
- McCoy MK, Martinez TN, Ruhn KA, et al. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci 2006;26:9365-75. [Crossref] [PubMed]
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener 2015;4:19. [Crossref] [PubMed]
- Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 2013;33:183-9. [Crossref] [PubMed]
- Menza M, Dobkin RD, Marin H, et al. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson's disease. Psychosomatics 2010;51:474-9. [PubMed]
- Scalzo P, Kümmer A, Cardoso F, Teixeira AL. Serum levels of interleukin-6 are elevated in patients with Parkinson's disease and correlate with physical performance. Neurosci Lett 2010;468:56-8. [Crossref] [PubMed]
- Veselý B, Dufek M, Thon V, et al. Interleukin 6 and complement serum level study in Parkinson's disease. J Neural Transm (Vienna) 2018;125:875-81. [Crossref] [PubMed]
- Diaz K, Kohut ML, Russell DW, et al. Peripheral inflammatory cytokines and motor symptoms in persons with Parkinson's disease. Brain Behav Immun Health 2022;21:100442. [Crossref] [PubMed]
- Blum-Degen D, Müller T, Kuhn W, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett 1995;202:17-20. [Crossref] [PubMed]
- Mogi M, Harada M, Narabayashi H, et al. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and transforming growth factor-α levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 1996;211:13-6. [Crossref] [PubMed]
- Nagatsu T, Mogi M, Ichinose H, et al. Cytokines in Parkinson's disease. J Neural Transm Suppl 2000;143-51. [PubMed]
- Borm CDJM, Werkmann M, de Graaf D, et al. Undetected ophthalmological disorders in Parkinson's disease. J Neurol 2022;269:3821-32. [Crossref] [PubMed]
- Graue-Hernández EO, Serna-Ojeda JC, Estrada-Reyes C, et al. Dry eye symptoms and associated risk factors among adults aged 50 or more years in Central Mexico. Salud Publica Mex 2018;60:520-7. [Crossref] [PubMed]
- Reddy VC, Patel SV, Hodge DO, et al. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea 2013;32:631-5. [Crossref] [PubMed]
- Tamer C, Melek IM, Duman T, et al. Tear film tests in Parkinson's disease patients. Ophthalmology 2005;112:1795. [Crossref] [PubMed]
- Massingale ML, Li X, Vallabhajosyula M, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009;28:1023-7. [Crossref] [PubMed]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007;14:1189-97. [Crossref] [PubMed]
- Mogi M, Togari A, Kondo T, et al. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm (Vienna) 2000;107:335-41. [Crossref] [PubMed]
- Mogi M, Harada M, Riederer P, et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 1994;165:208-10. [Crossref] [PubMed]
- Hirsch EC, Hunot S, Damier P, et al. Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann Neurol 1998;44:S115-20. [Crossref] [PubMed]
- Çomoğlu SS, Güven H, Acar M, et al. Tear levels of tumor necrosis factor-alpha in patients with Parkinson's disease. Neurosci Lett 2013;553:63-7. [Crossref] [PubMed]
- Martinez-Martin P, Rodriguez-Blazquez C, Alvarez-Sanchez M, et al. Expanded and independent validation of the Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS). J Neurol 2013;260:228-36. [Crossref] [PubMed]
- Aguilar-Navarro SG, Mimenza-Alvarado AJ, Palacios-García AA, et al. Validity and Reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the Detection of Cognitive Impairment in Mexico. Rev Colomb Psiquiatr (Engl Ed) 2018;47:237-43. [Crossref] [PubMed]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42. [Crossref] [PubMed]
- Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord 2013;28:668-70. [Crossref] [PubMed]
- Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 2011;151:792-798.e1. [Crossref] [PubMed]
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640-50. [Crossref] [PubMed]
- Söğütlü Sarı E, Koç R, Yazıcı A, et al. Tear Osmolarity, Break-up Time and Schirmer's Scores in Parkinson's Disease. Turk J Ophthalmol 2015;45:142-5. [Crossref] [PubMed]
- Brych M, Murali S, Händel B. How the motor aspect of speaking influences the blink rate. PLoS One 2021;16:e0258322. [Crossref] [PubMed]
- Martinez JD, Galor A, Amescua G, et al. Transcultural validation of the 5-Item Dry Eye Questionnaire for the Mexican population. Int Ophthalmol 2019;39:2313-24. [Crossref] [PubMed]
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf 2017;15:539-74. [Crossref] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95. [Crossref] [PubMed]
- Miller DB, O'Callaghan JP. Biomarkers of Parkinson's disease: present and future. Metabolism 2015;64:S40-6. [Crossref] [PubMed]
- Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J 2016;7:15. [Crossref] [PubMed]
- Börger M, Funke S, Bähr M, et al. Biomarker sources for Parkinson’s disease: Time to shed tears? Basal Ganglia 2015;5:63-9. [Crossref]
- Chen H, O'Reilly EJ, Schwarzschild MA, et al. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol 2008;167:90-5. [Crossref] [PubMed]
- Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson's disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord 2007;22:1852-6. [Crossref] [PubMed]
- Ton TG, Jain S, Biggs ML, et al. Markers of inflammation in prevalent and incident Parkinson's disease in the Cardiovascular Health Study. Parkinsonism Relat Disord 2012;18:274-8. [Crossref] [PubMed]
- Muzio L, Viotti A, Martino G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front Neurosci 2021;15:742065. [Crossref] [PubMed]
- Leal MC, Casabona JC, Puntel M, et al. Interleukin-1β and tumor necrosis factor-α: reliable targets for protective therapies in Parkinson's Disease? Front Cell Neurosci 2013;7:53. [Crossref] [PubMed]
- Kim SM, Song J, Kim S, et al. Identification of peripheral inflammatory markers between normal control and Alzheimer's disease. BMC Neurol 2011;11:51. [Crossref] [PubMed]
- Williams-Gray CH, Wijeyekoon R, Yarnall AJ, et al. Serum immune markers and disease progression in an incident Parkinson's disease cohort (ICICLE-PD). Mov Disord 2016;31:995-1003. [Crossref] [PubMed]
- Fu J, Chen S, Liu J, et al. Serum inflammatory cytokines levels and the correlation analyses in Parkinson's disease. Front Cell Dev Biol 2023;11:1104393. [Crossref] [PubMed]
- Gupta V, Garg RK, Khattri S. Levels of IL-8 and TNF-α decrease in Parkinson's disease. Neurol Res 2016;38:98-102. [Crossref] [PubMed]
- Ahmadi Rastegar D, Ho N, Halliday GM, et al. Parkinson's progression prediction using machine learning and serum cytokines. NPJ Parkinsons Dis 2019;5:14. [Crossref] [PubMed]
- Rentzos M, Nikolaou C, Andreadou E, et al. Circulating interleukin-10 and interleukin-12 in Parkinson's disease. Acta Neurol Scand 2009;119:332-7. [Crossref] [PubMed]
- Porro C, Cianciulli A, Panaro MA. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020;10:1017. [Crossref] [PubMed]
- Boka G, Anglade P, Wallach D, et al. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett 1994;172:151-4. [Crossref] [PubMed]
- Biousse V, Skibell BC, Watts RL, et al. Ophthalmologic features of Parkinson's disease. Neurology 2004;62:177-80. [Crossref] [PubMed]
- Nagino K, Sung J, Oyama G, et al. Prevalence and characteristics of dry eye disease in Parkinson's disease: a systematic review and meta-analysis. Sci Rep 2022;12:18348. [Crossref] [PubMed]
- Fitzpatrick E, Hohl N, Silburn P, et al. Case-control study of blink rate in Parkinson's disease under different conditions. J Neurol 2012;259:739-44. [Crossref] [PubMed]
- Lam H, Bleiden L, de Paiva CS, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol 2009;147:198-205. e1.
- Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci 2004;45:4293-301. [Crossref] [PubMed]
- Trousdale MD, Zhu Z, Stevenson D, et al. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J Autoimmune Dis 2005;2:6. [Crossref] [PubMed]
- Zhu Z, Stevenson D, Schechter JE, et al. Prophylactic effect of IL-10 gene transfer on induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci 2004;45:1375-81. [Crossref] [PubMed]
Cite this article as: Camacho-Ordonez A, Robles-Contreras A, Guerrero-Berger O, Camacho-Ordonez N, Rodríguez-Rivas R, Adalid-Peralta L, Cervantes-Arriaga A, Rodríguez-Violante M. Pro-inflammatory cytokines levels in tears and dry eye disease in Parkinson’s disease. Ann Eye Sci 2023;8:16.