
Comprehensive and updated review on the diagnosis and treatment of Vogt-Koyanagi-Harada disease
Introduction
Background
Vogt-Koyanagi-Harada (VKH) syndrome is a rare progressive inflammatory condition that presents with bilateral granulomatous panuveitis and a constellation of neurologic, auditory, and integumentary manifestations. The name of the syndrome is taken after Alfred Vogt, a Swiss physician who described the first case in the literature, and Japanese researchers Yoshizo Koyanagi and Einosuke Harada who comprehensively described the syndrome and its clinical course in the early twentieth century (1).
Early ocular manifestations of VKH include multi-focal serous retinal detachments (SRDs) and choroidal thickening, which later develop into granulomatous anterior uveitis and progressive posterior segment depigmentation, also known as “sunset glow fundus”. Systemic manifestations range from nonspecific symptoms resembling a viral illness during the initial onset to hearing loss, poliosis, vitiligo, and alopecia in the chronic stage (2). VKH can occur at any age but the onset is typically in the middle-age, and appears to affect women slightly more than men (3). It is highly prevalent in Asians, accounting for 7% of all ocular inflammatory conditions in Japan and 15.9% in China, but has been reported less frequently in Caucasians or blacks (4-6).
The diagnosis for VKH is challenging due to ocular and extraocular manifestations that occur at different stages of the disease and a broad differential, which includes infectious and noninfectious uveitis (NIU), intraocular lymphoma, and rare conditions like sympathetic ophthalmia (SO). The evolution of the diagnostic criteria over the last few decades reflects our growing understanding of the clinical features and disease progress of VKH (7-9). The pathogenesis of VKH is still not known, but there is evidence that it involves a T-cell mediated immune response against antigens on melanocytes in genetically susceptible individuals, such as those with human leukocyte antigen (HLA) subtype DR4/DRB1*04 (10).
Rationale and knowledge gap
There is limited information to guide ophthalmologists in the management of VKH, particularly regarding the use of newer imaging modalities and corticosteroid-sparing immunomodulatory therapy (IMT) in the acute and chronic stages of VKH.
Objective
The primary focus of this review is to provide a summary of the clinical features of VKH in the context of newer imaging modalities, the evolution of the diagnostic criteria, and the new studies on immunosuppression, in order to assist clinicians in their diagnosis and treatment of patients with VKH.
Clinical features
The disease course of VKH can be categorized into four stages: prodromal, uveitis, convalescent, and recurrent. The prodromal stage lasts several days and involve symptoms of malaise, headache, nausea, and/or neck and back stiffness, which was reported in about 50% of patients with VKH (2,11). In the uveitis stage, patients typically develop sudden onset of blurry vision in one or both eyes. Clinical exam findings include bilateral granulomatous panuveitis with predominantly posterior involvement, choroidal thickening, swelling and hyperemia of the optic nerve, and SRD (Figure 1) (12). SRD is the most common and specific feature of acute VKH and had a positive predictive value (PPV) of 100 and negative predictive value (NPV) of 88.4 in a cohort of patients presenting with bilateral ocular inflammation (13).
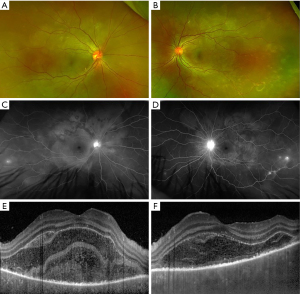
The convalescent stage occurs several weeks after the onset of acute uveitis stage and is characterized by depigmentation of the fundus, or “sunset glow fundus.” This feature is less frequently reported in Caucasians, perhaps due to challenges discerning features of depigmentation against a less pigmented fundus (13). Interestingly, a study found higher levels of pleocytosis in patients who eventually developed sunset glow fundus, which suggests an association between the severity of inflammation with developing chronic signs of VKH (14). Nummular chorioretinal scars are also common in the late stage of VKH and were reported in over 75% of patients who were examined several weeks after disease onset (15). Another form of depigmentation that is seen in the early chronic stage is perilimbal vitiligo, or “Sugiura’s sign”, found in up to 85% of Japanese patients with VKH but less frequently in studies without a predominant have Japanese cohort (2,13,16).
The chronic recurrent stage is characterized by subclinical or recurrent episodes of granulomatous anterior uveitis and is associated with complications from chronic intraocular inflammation. The most common complications of chronic VKH include cataracts, possibly due to prolonged systemic corticosteroid therapy, followed by glaucoma, and subretinal fibrosis (17). Sunset glow fundus remains a distinguishing feature of chronic VKH, reported in 68% of Japanese patients with ocular inflammation lasting for more than 6 months (14). Overall, the intraocular inflammation in VKH follows a predictable course that starts with a predominantly posterior uveitis and evolves into an anterior granulomatous uveitis during the chronic stage (18).
Extraocular manifestations in VKH follow a less predictable course and can variably affect the auditory, integumentary, and neurologic systems, typically in the late stage of VKH. The most common systemic manifestations include headaches and tinnitus, reported in 49% and 36% of patients with VKH, respectively (13). Systemic manifestations in VKH have also been reported to vary in different populations. Native American patients with VKH reported significantly less frequent systemic symptoms compared to non-Native Americans, but integumentary manifestations like vitiligo were more common (19). Hispanic patients with VKH also have fewer systemic manifestations, including tinnitus, which was reported in 10–15% of patients (2,20).
Multimodal imaging
Fundus autofluorescence (FAF) photography
FAF photography is a noninvasive imaging modality used to visualize lipofuscin, a byproduct of lysosomal breakdown of retinal pigment epithelium (RPE), which can help detect subtle damage or loss of RPE in VKH. In the acute stage of VKH, SRD can be visualized as hypoautofluorescence in FAF due to blockage. Resolution of SRD can leave behind granular hypoautofluorescent dots corresponding to hyperfluorescent dots on fluorescein angiography (FA), representing damaged or missing RPE (21). There are no direct signs on FAF for sunset glow fundus, but lesions with decreased autofluorescence on FAF are seen in a majority of patients with chronic VKH and correspond to loss of RPE and outer retinal involvement on optical coherence tomography (OCT) (22). Hyperautofluorescent lesions on FAF are seen in both acute and chronic VKH and can represent a wide range of RPE abnormalities, including RPE hyperplasia, subretinal fibrosis, loss of inner or outer segment junction, or cystoid macular edema (22,23).
OCT
Spectral domain optical coherence tomography (SD-OCT) can be used to visualize and monitor the SRDs and subretinal fluid in patients with acute VKH (24). A distinguishing feature of SRD in VKH is the multiple cystic space that is separated by hyperreflective septa, which has been hypothesized as inflammatory fibrinous material due to its disappearance with corticosteroid treatment (25,26). In a recent study, this fibrinous membrane was found in 61.5% of eyes in patients with initial-onset VKH and was associated with younger age and shorter time from the onset of symptoms, which suggests that it is an early feature of SRD in patients with acute VKH (27).
Swept-source OCT (SS-OCT) is the latest generation of OCT imaging that has a faster scanning speed and uses a longer wavelength than conventional SD-OCT imaging, resulting in better visualization of the deeper layers of the retina and choroid. Recently, Agarwal and colleagues used SS-OCT to evaluate 62 patients with acute VKH and found that 95% of eyes with SRD had a split at the photoreceptor myoid, leaving behind a bacillary layer consisting of the remaining myoid, ellipsoid zone (EZ) and interdigitation zone (IZ) (28). This is called “bacillary layer detachment” and has been described in SRD associated with toxoplasma retinochoroiditis, tubercular choroidal granuloma, and other inflammatory diseases (29,30).
Enhanced depth imaging (EDI) is a method of SD-OCT that can visualize the deeper structures of the eye and has been used to characterize the choroid in the acute and chronic stages of VKH (31). Thickened choroid on EDI-OCT is a common feature of acute VKH and was incorporated into the diagnostic criteria that was recently developed by Yang and colleagues (8). On the other hand, the thickness of the choroid in patients with convalescent VKH is comparable to that of control subjects, which is likely a result of ongoing inflammation and loss of choroidal structure (32). In a study by Nakayama and colleagues, EDI-OCT detected rebound choroidal thickening in patients during corticosteroid tapering in the absence of clinical recurrence (33). Subclinical inflammation can go unnoticed in patients with VKH, which can lead to chronic disease and irreversible loss of visual acuity. EDI-OCT is a quick and noninvasive imaging test that can be used to monitor the disease status and treatment response in patients presenting with thickened choroid in the acute stage of VKH.
FA and indocyanine green angiography (ICGA)
FA findings in VKH have been well described in the literature. In the acute stage, optic disc hyperfluorescence and early pinpoint areas of hyperfluorescence followed by late pooling in SRD can be seen on FA (Figure 1C,1D). In the chronic stage, common findings include optic disc hyperfluorescence and spotted hyper and hypofluorescence, or “salt and pepper” fundus appearance, representing RPE damage (34). SRD in VKH appears as multi-lobular pooling of dye within the subretinal space on late-phase FA (26). In patients with acute VKH but without SRD, delayed choroidal perfusion, optic disc hyperfluorescence, mild pinpoint leakage, and choroidal folds can be seen on the FA (24).
ICGA provides enhanced visualization of the choroidal circulation and can detect subtle choroidal inflammation that is not detected by FA. Herbort and colleagues identified major ICGA signs in patients with acute VKH, including early choroidal stromal vessel hyperfluorescence and leakage, hypofluorescent dark dots, fuzzy vascular pattern of large stromal vessels, and disc hyperfluorescence. Of these, hypofluorescent dark dots, representing choroidal inflammation, were the most prominent and resolved in a majority of patients who underwent treatment (35). In another study, the hypofluorescent dark dots resolved with high-dose but not medium-dose corticosteroids in patients with clinical improvement of VKH (36). ICGA-guided therapy has been shown to prevent progression to chronic disease in a small number of patients who were managed more aggressively based on subtle findings of choroidal recurrence on ICGA (37). Interestingly, a study by Chee and colleagues found early pinpoint peripapillary hyperfluorescence on the FA was associated with a good prognosis in patients, whereas ICGA findings did not have prognostic value (38).
Diagnostic criteria
The diagnostic criteria for VKH syndrome have been modified several times in the last few decades. In 1978, Sugiura published a set of criteria for the diagnosis of VKH, which requires (I) acute bilateral uveitis, (II) retinal edema with typical FA findings, and (III) pleocytosis of the cerebrospinal fluid (CSF) noted in early stage of the disease. The diagnostic criteria did not consider any other systemic manifestations involving the auditory or integumentary system and excluded a significant proportion of patients with VKH who did not have CSF pleocytosis, which is a common but not universal finding in patients with VKH (39).
The diagnostic criteria by the American Uveitis Society (AUS), also published in 1978, added a criterion to differentiate VKH syndrome from SO, which can present with similar findings on exam and imaging. In addition, at least one finding from three of the following categories is required: (I) bilateral chronic iridocyclitis; (II) posterior uveitis; (III) neurologic signs; and (IV) cutaneous signs. A major criticism of the AUS diagnostic criteria is the lack of consideration of the different stages of VKH, since posterior uveitis occurs in the acute stage while iridocyclitis and systemic manifestations are more common in the chronic stage of VKH.
In 2001, the revised diagnostic criteria were published by experts after the First International Workshop on VKH in 1999. The criteria categorize VKH into complete, incomplete, and probable VKH according to the number of systems involved. The ocular manifestations are differentiated according to the stage of the disease and incorporate findings from FA and ultrasonography. The revised diagnostic criteria have been reported to capture 90% of patients diagnosed with VKH with Sugiura’s criteria and 100% of patients who were diagnosed with VKH based on the clinicians’ expertise (15,39). In a study evaluating the revised diagnostic criteria, Kitamura and colleagues reported two patients with bilateral disc edema, inflammatory cells in the AC, and CSF pleocytosis, who were not diagnosed with VKH due to the absence of SRD. The presence of focal subretinal fluid or SRD is central to the diagnosis of early VKH in the revised diagnostic criteria, since without either, characteristic findings must be present in both FA and ultrasonography for the diagnosis of VKH. Yamaki and colleagues also evaluated the revised diagnostic criteria in a group of 49 patients and determined that the revised diagnostic criteria would not adequately capture patients in the early stage of the disease (40). However, the study did not include patients with ‘probable VKH,’ which leads to the question of whether this conclusion would still hold had this category been included. Overall, the revised diagnostic criteria are well-validated for VKH and appear to be the most frequently used in the clinical and research setting.
In 2018, Yang and colleagues published a new set of diagnostic criteria by retrospectively analyzing data from more than 1,100 patients with VKH and 1,100 patients with non-VKH associated uveitis (8). Patients are categorized into early or late phase of the disease as well as variant 1, 2, or 3 depending on the type of ocular manifestations. The criteria also incorporate findings of choroidal thickening from the EDI-OCT, achieving a higher sensitivity and NPV than the revised diagnostic criteria from 2001. However, critiques pointed out that PPV and NPV are actually lower in real populations since the prevalence is artificially set at 50% in the study (41). One of the criteria, “no evidence of infectious uveitis or accompanying systemic rheumatic diseases or evidence suggestive of other ocular disease entities,” was also criticized as it is broad catch-all statement that would leave VKH as the default diagnosis, not to mention require extensive workup.
In 2021, a classification criteria for VKH was developed by The SUN Working Group by applying machine learning on 1,012 cases of panuveitis, including 156 cases of early-stage VKH and 103 cases of late-stage VKH (9). The classification criteria, different from diagnostic criteria, optimized for specificity instead of sensitivity because the purpose of the diagnosis is for research. Therefore, clinicians should not use these criteria to diagnose patients with VKH and only use the data as reference.
Finally in 2022, Herbort and colleagues proposed a simplified diagnostic criteria for acute initial-onset VKH, in which bilateral disease and diffuse choroiditis on EDI-OCT or ICGA are requirements for the diagnosis of VKH (12). Authors emphasized the usefulness of ICGA in the early detection of VKH in the choroid, particularly in subclinical disease with choroidal involvement that is not evident on clinical exam or FA, and recommended EDI-OCT in settings without access to ICGA. No alternative options were provided to verify the presence of bilateral vitritis in the absence of ICGA or EDI-OCT. Other required criteria include the absence of ocular trauma or surgery before disease, bilateral involvement, exclusion of other infectious, inflammatory or masquerading entities, as well as criteria to exclude chronic VKH. In addition, exudative RD is listed as a “very helpful criterion” and disc hyperfluorescence and neurological or auditory findings as “helpful criterion”. However, there are no specifications on how these “helpful criteria” actually contribute to the diagnosis of VKH, especially in patients who don’t meet all the required criteria.
Differential diagnosis
The diagnosis of VKH is challenging partly due to the broad differential, which includes infectious and noninfectious causes of uveitis, malignancy, and other rare ocular inflammatory conditions. A study reported that the mean time to diagnosis in VKH was 2.5 years due to a number of misdiagnoses, which highlights the importance of understanding the similarities and differences of the top differentials in VKH (11).
Infectious cause of uveitis
Infectious causes of uveitis should be suspected in patients with recent intraocular surgery, trauma, exposure to sexually transmitted diseases, or travel to countries with endemic diseases such as tuberculosis. Intraocular tuberculosis frequently presents with posterior and panuveitis involving both eyes, which can be difficult to differentiate from VKH without systemic involvement (42). Work up includes interferon-gamma release assay (IGRA) or tuberculin skin test (TST) in conjunction with chest imaging to rule out active disease (43). Uveitis is the most common presentation of ocular syphilis, of which granulomatous and non-granulomatous iridocyclitis is reported in 71% patients (44). A treponemal test, such as T.pallidum passive particle agglutination (TP-PA) assay, fluorescent treponemal antibody absorption test (FTA-ABS), or microhemagglutination-T.pallidum Test (MHA-TA), is ordered with a non-treponemal test, such as Venereal Disease Research Laboratory (VDRL) or rapid plasma reagent (RPR) (45).
Noninfectious cause of uveitis
Ocular involvement is seen in 25–60% of patients with sarcoidosis, of which uveitis is the most common ocular manifestation (46). Anterior uveitis is frequently reported in ocular sarcoidosis and can present as chronic granulomatous uveitis, which is similar to VKH in the chronic stage. If ocular sarcoidosis is suspected, patients should be screened for pulmonary disease with chest radiograph and a biopsy should be obtained if necessary. Systemic lupus erythematous (SLE) usually manifest with ocular surface disease or retinopathy, but can rarely present with choroidopathy with serous detachments (47). A study reported increased thickness of choroid using EDI-OCT in patients with SLE, but this was not consistently demonstrated in other studies. The diagnosis of SLE requires a constellation of systemic and immunologic manifestations, but a positive antinuclear antibodies was found to have sensitivity of 99.5% (48).
Vitreoretinal lymphoma (VRL)
VRL, also known as primary intraocular lymphoma, is a type of non-Hodgkin’s lymphoma and is the most common intraocular lymphoma. VRL involves the central nervous system in a majority of patients and is a fatal disease with a mean survival of 32 months (49). Patients usually complain of blurred vision or floaters and are found to have posterior uveitis with vitritis or white or yellow raised subretinal lesions, which can be visualized as subretinal or sub-RPE deposits on SD-OCT (50). Older patients with chronic uveitis that is refractory to immunosuppressive therapy should be worked up for possible malignancy with vitreoretinal biopsy if neuroimaging or lumbar puncture are not diagnostic.
SO
SO is as a rare bilateral granulomatous panuveitis that occurs after penetrating ocular trauma or intraocular surgery in one eye, and can present with similar clinical features as VKH, such as bilateral panuveitis, choroiditis, retinal detachment, and sunset glow fundus (18). Although the exact pathogenesis is not known, like VKH, the leading hypothesis involves a T-cell response against the exposed antigens of the photoreceptor layer and melanocytes (51). SO can usually be differentiated from VKH with a history of penetrating ocular trauma or intraocular surgery but can also be differentiated by the clinical features. The evolution of intraocular disease in VKH is predictable—panuveitis dominates in the acute stage of the disease, eventually involving the anterior segment, and finally manifest as recurrent granulomatous anterior uveitis. SO does not progress in a predictable way and can present with anterior or posterior uveitis at different stages of the disease. Systemic manifestations such as meningismus, tinnitus, alopecia vitiligo are also more common in VKH than SO (18).
Posterior scleritis (PS)
PS is rare inflammatory condition and patients typically present with headache and vision loss. Bilateral disease is seen in 35% and SRD in 21% of patients with PS (52). A study comparing imaging findings between PS and VKH found that hyperreflective dots, multiple SRD, and RPE fold were common in patients with VKH while single SRD was more common in the OCT of patients with PS. The ‘T-sign’ on ultrasonography, representing fluid accumulation in the subtenon space, was specific for PS and present in about 33% of patients (53). Additionally, systemic manifestations are more common in VKH and recurrence manifest as anterior uveitis in VKH in contrast to posterior segment inflammation in PS.
Central serous chorioretinopathy (CSC)
CSC is a disorder that is characterized by SRD and bilateral cases can present similarly to VKH. A study reported that 90 out of 410 or about 22% of patients with VKH were misdiagnosed with CSC (11). A close comparison of FA and ICGA findings between VKH and CSC showed that disc leakage and hypofluorescent dots were more common in VKH while multifocal leakage and choroidal vascular hyperpermeability were more common in CSC. OCT showed pigment epithelial detachment in 60% of patients with CSC, which was not observed in any of the patients with VKH. Subretinal fluid, subretinal septa, and RPE folds were more common in VKH patients on OCT (54).
Treatment
Systemic corticosteroids
The mainstay treatment for VKH is high-dose systemic corticosteroids with prolonged tapering. IMT is typically added to the treatment regimen if the inflammation persists or becomes worse while on corticosteroid therapy (55). No difference in visual outcomes has been reported between initial treatment with oral or intravenous corticosteroids (56). However, rapid tapering of corticosteroids in less than 6 months has been associated with worse visual outcomes and recurrence of inflammation (57-59). Early treatment with corticosteroids is an important prognostic factor and has been associated with better visual outcomes and less persistent inflammation (60). The “therapeutic window” or “window of opportunity” represents an early time period in a disease during which treatment can lead to optimal clinical outcomes, and has been shown to be important in autoimmune diseases like rheumatoid arthritis and increasingly so in VKH (61). Chee and colleagues found that 66% of patients who received high-dose corticosteroids within the first two weeks of disease onset had complete resolution of inflammation whereas all of the patients who received treatment within two to four weeks developed chronic or recurrent disease (62). Yang and colleagues identified treatment after two weeks of disease onset was associated with worse final BCVA (63). Based on the results of these studies, early initiation of corticosteroids is key to visual and inflammatory outcomes (Figure 2).
IMT
There is growing evidence that systemic corticosteroids may not be sufficient to prevent subclinical inflammation and development of chronic disease, even in a patient who had early initiation of corticosteroids. According to a systematic review including 16 studies and 802 patients with initial acute onset VKH, 44% had recurrent disease after treatment with high-dose corticosteroids (64). Sakata and colleagues reported a staggering 79% recurrence rate in Brazilian patients with VKH who were treated with high-dose corticosteroids within 1 month of disease onset (65). Other studies reported a smaller but a significant rate of recurrence despite early treatment with high-dose corticosteroids (62,66). Although some of the recurrence may have been prevented with an earlier initiation of corticosteroids, the high rate of recurrence suggests that there is a subset of patients with VKH that requires additional therapy with IMT.
Several studies support early initiation of IMT and even using it as a first-line treatment with corticosteroids. Yang and colleagues evaluated the effect of reduced corticosteroids in combination with one or more IMT, such as azathioprine, methotrexate, cyclosporine and cyclophosphamide, which showed that 98% of almost 1,000 patients with VKH achieved remission of uveitis (63). Urzua and colleagues reported that patients with poor glucocorticoid response had visual improvement associated with earlier initiation of IMT (67). Similarly, other studies reported better visual outcomes and remission in patients with chronic VKH when IMT was initiated early (28,68,69).
Azathioprine
Azathioprine (Imuran, Promethus Labs, Inc., San Diego, CA, USA) is an antimetabolite that is often used to treat chronic NIU. Kim and colleagues investigated the effect of azathioprine and systemic corticosteroids in a small number of patients with acute and chronic VKH, which revealed that while the addition of azathioprine resulted in corticosteroid-sparing effects, there was no significant difference in the cumulative corticosteroid dose, visual acuity, recurrence rate, or complications compared to patients who only received corticosteroids (70). Comparison of prednisone with azathioprine or cyclosporine showed that both regimen resulted in improved visual acuity and inflammation in patients with chronic VKH, but azathioprine had less glucocorticoid-sparing effect than cyclosporine (71).
Methotrexate
Methotrexate (Rheumatrex, Dava Pharmaceuticals, Inc. Newark, DE, and others) is another antimetabolite that is frequently used to treat chronic NIU. Kondo and colleagues reported three cases in which methotrexate was used in conjunction with systemic steroids to successfully control intraocular inflammation in patient with VKH, allowing two of the patients to stop systemic corticosteroids (72). It was also shown to control inflammation in seven out ten pediatric patients with acute VKH who were intolerant or unresponsive to initial treatment with systemic corticosteroids (73).
Mycophenolate Mofetil
Mycophenolate Mofetil (MMF; Cellcept, Genentech, Inc., San Francisco, CA, USA) is an antimetabolite used to treat chronic NIU and has been of primary interest to Abu El-Asrar and colleagues, who investigated the use of MMF as first-line treatment in patients with acute VKH. Their study showed that patients who received MMF in addition to systemic corticosteroids had better visual acuity as well as reduced rates of recurrence and complications compared to those who only received systemic corticosteroids (74). In patients with initial acute onset VKH, first-line treatment with MMF and systemic corticosteroids resulted in remission of disease in all patients with a visual acuity of 20/20 in 93.4% of eyes (75). Finally, a subanalysis of VKH patients from the First-line Antimetabolites as Steroid-sparing Treatment (FAST) trial on NIU showed that treatment with MMF or Methotrexate with systemic corticosteroids led to resolution of inflammation in a majority of patients (66%) with no statistical significant difference in efficacy between the two therapies (74% Methotrexate vs. 53% Mycophenolate Mofetil) (76).
Cyclosporine
Cyclosporine (Neoral Novartis Pharmaceuticals Corp., New York, NY, USA) is a calcineurin inhibitor that has been used to treat various NIU entities with varying efficacy (77). A study in Japan reported cyclosporine as the most common first choice IMT for patients with early and late stage VKH, but 5 out of 21 patients had recurrence and 14 patients had to discontinue due to side effects (78). A small case control study showed that a majority of patients with chronic VKH had remission of disease after a 3-month treatment with low-dose cyclosporine (79). Cyclosporine with prednisone improved visual acuity and aqueous flare in patients with chronic VKH and also had greater glucocorticoid-sparing effect compared to azathioprine and prednisone in a very small randomized controlled trial (N=21) (71).
Adalimumab
Adalimumab (Humira, AbbVie, Inc., North Chicago, IL, USA) is a human monoclonal antibody that is directed against tumor necrosis factor (TNF)-α and is the first-line therapy for Behçet disease and second-line for juvenile idiopathic arthritis-associated uveitis (80). It is the only FDA-approved IMT for the treatment of non-infectious intermediate, posterior and panuveitis (81). Adalimumab was used to treat chronic recurrent VKH in several studies with mixed results. The largest study included 70 patients with chronic VKH who had failed therapy with IMT such as azathioprine, cyclosporine, and methotrexate in conjunction with high-dose corticosteroids. Patients in the study received over 6 months of adalimumab therapy and had improvement of subfoveal choroidal thickness and ICGA scores, leading to reduction of corticosteroids and cyclosporine doses. There were no significant improvements in visual acuity or flare counts, but a subset of patients with sunset glow fundus were found to have significant improvement in visual acuity after adalimumab treatment (82). Yang and colleagues evaluated the clinical outcomes of nine patients with chronic VKH who received an average of 10 months of adalimumab treatment, which showed improvement in BCVA, AC cell and vitritis but patients were not completely relapse free (83). In a study by Hiyama and colleagues, 11 out of 14 patients who received adalimumab as the only IMT had recurrence of inflammation and required the addition of methotrexate. While methotrexate as the only IMT also had high recurrence rates, the combination of adalimumab and methotrexate controlled the inflammation and eight out of 11 patients achieved remission of disease (78). A few case reports have showed clinical improvement and remission of disease in patients who received adalimumab therapy, two of which were on pediatric patients (84-86).
Infliximab
Infliximab (Remicade, Janssen Biotechn, Inc., Titusville, NJ, USA) is a chimeric monoclonal antibody against TNF-α with limited studies on treatment for VKH. There have only been case reports on the use of infliximab to treat VKH, which generally reported good visual outcomes and remission of disease (87-91). Methotrexate was often used in conjunction to prevent the formation of anti-infliximab antibodies (88-91). Of the three cases reported on the use of infliximab in pediatric patients, a 12 year-old Hispanic girl had persistent SRD and submacular fluid on infliximab that improved on cyclophosphamide treatment (91,92).
Rituximab
Rituximab (Rituxan, Genentech, South San Francisco, CA, USA) is a monoclonal antibody to CD20 on B cells that has been studied in a small number of patients with VKH. In one study, nine patients with chronic recurrent VKH achieved remission after treatment with three infusions of Rituximab (92). Improvement in vision and choroidal thickness on EDI-OCT was also observed in five patients with poorly controlled VKH after infusions with rituximab (93). A ten-year-old girl had improved vision and remission of disease and another young patient had recovery of high-frequency hearing loss after treatment with rituximab (94,95).
Conclusions
VKH follows a predictable disease course that starts with an acute bilateral panuveitis and evolves into a granulomatous anterior uveitis in the chronic stage. The acute stage of VKH is characterized by bilateral SRD while the chronic stage is characterized by sunset glow fundus. The SRDs in VKH contain multiple cystic spaces, which has been hypothesized as fibrinous septa but was recently shown to be associated with “bacillary layer detachment”, or a split in the myoid, on SS-OCT. Other new imaging modalities such as EDI-OCT and ICGA were shown to be useful in detecting subclinical inflammation, which likely contributes to recurrence and progression to chronic disease in VKH.
While there are several renditions of the diagnostic criteria for VKH, the revised diagnostic criteria are the most well-validated. Rare ocular inflammatory conditions including SO, PS, and CSC should be considered in the differential diagnosis of VKH in addition to infectious and noninfectious causes of uveitis. For the treatment of VKH, studies showed that the early initiation of high-dose systemic corticosteroids was associated with better visual acuity outcomes and decrease rates of recurrence, but a subset of patients still developed recurrence or chronic disease. Studies on IMT show promising results and a combination therapy with corticosteroids has the potential to treat patients with VKH that did not respond to monotherapy with corticosteroids.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kareem Moussa) for the series “The Retina and Systemic Disease” published in Annals of Eye Science. The article has undergone external peer review.
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-23-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-23-3/coif). The series “The Retina and Systemic Disease” was commissioned by the editorial office without any funding or sponsorship. JGS has received small honoraria for a talk in March Berkley Optometry and CME writing activity Retina Specialist. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbort CP, Mochizuki M. Vogt-Koyanagi-Harada disease: inquiry into the genesis of a disease name in the historical context of Switzerland and Japan. Int Ophthalmol 2007;27:67-79. [Crossref] [PubMed]
- Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol 1995;39:265-92. [Crossref] [PubMed]
- Wang Y, Chan CC. Gender differences in vogt-koyanagi-harada disease and sympathetic ophthalmia. J Ophthalmol 2014;2014:157803. [Crossref] [PubMed]
- Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol 2007;55:173-83. [Crossref] [PubMed]
- Ohguro N, Sonoda KH, Takeuchi M, et al. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol 2012;56:432-5. [Crossref] [PubMed]
- Yang P, Zhang Z, Zhou H, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res 2005;30:943-8. [Crossref] [PubMed]
- Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 2001;131:647-52. [Crossref] [PubMed]
- Yang P, Zhong Y, Du L, et al. Development and Evaluation of Diagnostic Criteria for Vogt-Koyanagi-Harada Disease. JAMA Ophthalmol 2018;136:1025-31. [Crossref] [PubMed]
- Classification Criteria for Vogt-Koyanagi-Harada Disease. Am J Ophthalmol 2021;228:205-11. [Crossref] [PubMed]
- Shi T, Lv W, Zhang L, et al. Association of HLA-DR4/HLA-DRB1*04 with Vogt-Koyanagi-Harada disease: a systematic review and meta-analysis. Sci Rep 2014;4:6887. [Crossref] [PubMed]
- Yang P, Ren Y, Li B, et al. Clinical characteristics of Vogt-Koyanagi-Harada syndrome in Chinese patients. Ophthalmology 2007;114:606-14. [Crossref] [PubMed]
- Herbort CP Jr, Tugal-Tutkun I, Abu-El-Asrar A, et al. Precise, simplified diagnostic criteria and optimised management of initial-onset Vogt-Koyanagi-Harada disease: an updated review. Eye (Lond) 2022;36:29-43. [Crossref] [PubMed]
- Rao NA, Gupta A, Dustin L, et al. Frequency of distinguishing clinical features in Vogt-Koyanagi-Harada disease. Ophthalmology 2010;117:591-9, 599.e1.
- Keino H, Goto H, Mori H, et al. Association between severity of inflammation in CNS and development of sunset glow fundus in Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2006;141:1140-2. [Crossref] [PubMed]
- Rao NA, Sukavatcharin S, Tsai JH. Vogt-Koyanagi-Harada disease diagnostic criteria. Int Ophthalmol 2007;27:195-9. [Crossref] [PubMed]
- Ohno S, Minakawa R, Matsuda H. Clinical studies of Vogt-Koyanagi-Harada's disease. Jpn J Ophthalmol 1988;32:334-43. [PubMed]
- Read RW, Rechodouni A, Butani N, et al. Complications and prognostic factors in Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2001;131:599-606. [Crossref] [PubMed]
- Yang P, Liu S, Zhong Z, et al. Comparison of Clinical Features and Visual Outcome between Sympathetic Ophthalmia and Vogt-Koyanagi-Harada Disease in Chinese Patients. Ophthalmology 2019;126:1297-305. [Crossref] [PubMed]
- Reddy AK, John FT, Justin GA, et al. Vogt-Koyanagi-Harada disease in a Native American population in Oklahoma. Int Ophthalmol 2021;41:915-22. [Crossref] [PubMed]
- Sukavatcharin S, Tsai JH, Rao NA. Vogt-Koyanagi-Harada disease in Hispanic patients. Int Ophthalmol 2007;27:143-8. [Crossref] [PubMed]
- Ayata A, Dogru S, Senol MG, et al. Autofluorescence findings in Vogt-Koyanagi-Harada disease. Eur J Ophthalmol 2009;19:1094-7. [Crossref] [PubMed]
- Vasconcelos-Santos DV, Sohn EH, Sadda S, et al. Retinal pigment epithelial changes in chronic Vogt-Koyanagi-Harada disease: fundus autofluorescence and spectral domain-optical coherence tomography findings. Retina 2010;30:33-41. [Crossref] [PubMed]
- Koizumi H, Maruyama K, Kinoshita S. Blue light and near-infrared fundus autofluorescence in acute Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2010;94:1499-505. [Crossref] [PubMed]
- Attia S, Khochtali S, Kahloun R, et al. Clinical and multimodal imaging characteristics of acute Vogt-Koyanagi-Harada disease unassociated with clinically evident exudative retinal detachment. Int Ophthalmol 2016;36:37-44. [Crossref] [PubMed]
- Tsujikawa A, Yamashiro K, Yamamoto K, et al. Retinal cystoid spaces in acute Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 2005;139:670-7. [Crossref] [PubMed]
- Yamaguchi Y, Otani T, Kishi S. Tomographic features of serous retinal detachment with multilobular dye pooling in acute Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2007;144:260-5. [Crossref] [PubMed]
- Motegi S, Nagura K, Yoneda K, et al. Clinical relevance of fibrin membranous structures in the intra-photoreceptor outer segment separation on spectral domain optical coherence tomography (SD-OCT) in initial-onset acute Vogt-Koyanagi-Harada disease. Acta Ophthalmol 2023;101:e286-e293. [Crossref] [PubMed]
- Agarwal M, Ganesh SK, Biswas J. Triple agent immunosuppressive therapy in Vogt-Koyanagi-Harada syndrome. Ocul Immunol Inflamm 2006;14:333-9. [Crossref] [PubMed]
- Mehta N, Chong J, Tsui E, et al. Presumed foveal bacillary layer detachment in a patient with toxoplasmosis chorioretinitis and pachychoroid disease. Retin Cases Brief Rep 2021;15:391-8. [Crossref] [PubMed]
- Markan A, Aggarwal K, Gupta V, et al. Bacillary layer detachment in tubercular choroidal granuloma: A new optical coherence tomography finding. Indian J Ophthalmol 2020;68:1944-6. [Crossref] [PubMed]
- Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina 2011;31:510-7. [Crossref] [PubMed]
- Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina 2011;31:502-9. [Crossref] [PubMed]
- Nakayama M, Keino H, Watanabe T, et al. Clinical features and visual outcomes of 111 patients with new-onset acute Vogt-Koyanagi-Harada disease treated with pulse intravenous corticosteroids. Br J Ophthalmol 2019;103:274-8. [Crossref] [PubMed]
- Arellanes-García L, Hernández-Barrios M, Fromow-Guerra J, et al. Fluorescein fundus angiographic findings in Vogt-Koyanagi-Harada syndrome. Int Ophthalmol 2007;27:155-61. [Crossref] [PubMed]
- Herbort CP, Mantovani A, Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. Int Ophthalmol 2007;27:173-82. [Crossref] [PubMed]
- Kawaguchi T, Horie S, Bouchenaki N, et al. Suboptimal therapy controls clinically apparent disease but not subclinical progression of Vogt-Koyanagi-Harada disease. Int Ophthalmol 2010;30:41-50. [Crossref] [PubMed]
- Bouchenaki N, Herbort CP. Indocyanine green angiography guided management of vogt-koyanagi-harada disease. J Ophthalmic Vis Res 2011;6:241-8. [PubMed]
- Chee SP, Jap A, Cheung CM. The prognostic value of angiography in Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2010;150:888-93. [Crossref] [PubMed]
- Kitamura M, Takami K, Kitaichi N, et al. Comparative study of two sets of criteria for the diagnosis of Vogt-Koyanagi-Harada's disease. Am J Ophthalmol 2005;139:1080-5. Erratum in: Am J Ophthalmol 2006;141:1179. [Crossref] [PubMed]
- Yamaki K, Hara K, Sakuragi S. Application of revised diagnostic criteria for vogt-koyanagi-harada disease in Japanese patients. Jpn J Ophthalmol 2005;49:143-8. [Crossref] [PubMed]
- Jabs DA. Improving the Diagnostic Criteria for Vogt-Koyanagi-Harada Disease. JAMA Ophthalmol 2018;136:1032-3. [Crossref] [PubMed]
- Al-Mezaine HS, Al-Muammar A, Kangave D, et al. Clinical and optical coherence tomographic findings and outcome of treatment in patients with presumed tuberculous uveitis. Int Ophthalmol 2008;28:413-23. [Crossref] [PubMed]
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis 2017;64:111-5. [Crossref] [PubMed]
- Barile GR, Flynn TE. Syphilis exposure in patients with uveitis. Ophthalmology 1997;104:1605-9. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Syphilis - STI Treatment Guidelines [Internet]. 2022 [cited 2022 Nov 23]. Available online: https://www.cdc.gov/std/treatment-guidelines/syphilis.htm
- Rothova A. Ocular involvement in sarcoidosis. Br J Ophthalmol 2000;84:110-6. [Crossref] [PubMed]
- Lee I, Zickuhr L, Hassman L. Update on ophthalmic manifestations of systemic lupus erythematosus: pathogenesis and precision medicine. Curr Opin Ophthalmol 2021;32:583-9. [Crossref] [PubMed]
- Aringer M, Brinks R, Dörner T, et al. European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) SLE classification criteria item performance. Ann Rheum Dis 2021;80:775-81. [Crossref] [PubMed]
- Hong JT, Chae JB, Lee JY, et al. Ocular involvement in patients with primary CNS lymphoma. J Neurooncol 2011;102:139-45. [Crossref] [PubMed]
- Sagoo MS, Mehta H, Swampillai AJ, et al. Primary intraocular lymphoma. Surv Ophthalmol 2014;59:503-16. [Crossref] [PubMed]
- Fromal OV, Swaminathan V, Soares RR, et al. Recent advances in diagnosis and management of sympathetic ophthalmia. Curr Opin Ophthalmol 2021;32:555-60. [Crossref] [PubMed]
- McCluskey PJ, Watson PG, Lightman S, et al. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999;106:2380-6. [Crossref] [PubMed]
- Liu Z, Zhao W, Tao Q, et al. Comparison of the clinical features between posterior scleritis with exudative retinal detachment and Vogt-Koyanagi-Harada disease. Int Ophthalmol 2022;42:479-88. [Crossref] [PubMed]
- Shin WB, Kim MK, Lee CS, et al. Comparison of the Clinical Manifestations between Acute Vogt-Koyanagi-Harada Disease and Acute Bilateral Central Serous Chorioretinopathy. Korean J Ophthalmol 2015;29:389-95. [Crossref] [PubMed]
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000;130:492-513. [Crossref] [PubMed]
- Read RW, Yu F, Accorinti M, et al. Evaluation of the effect on outcomes of the route of administration of corticosteroids in acute Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2006;142:119-24. [Crossref] [PubMed]
- Rubsamen PE, Gass JD. Vogt-Koyanagi-Harada syndrome. Clinical course, therapy, and long-term visual outcome. Arch Ophthalmol 1991;109:682-7. [Crossref] [PubMed]
- Lai TY, Chan RP, Chan CK, et al. Effects of the duration of initial oral corticosteroid treatment on the recurrence of inflammation in Vogt-Koyanagi-Harada disease. Eye (Lond) 2009;23:543-8. [Crossref] [PubMed]
- Iwahashi C, Okuno K, Hashida N, et al. Incidence and clinical features of recurrent Vogt-Koyanagi-Harada disease in Japanese individuals. Jpn J Ophthalmol 2015;59:157-63. [Crossref] [PubMed]
- Chee SP, Jap A, Bacsal K. Prognostic factors of Vogt-Koyanagi-Harada disease in Singapore. Am J Ophthalmol 2009;147:154-161.e1. [Crossref] [PubMed]
- Hunt L, Buch M. The 'therapeutic window' and treating to target in rheumatoid arthritis. Clin Med (Lond) 2013;13:387-90. [Crossref] [PubMed]
- Chee SP, Jap A, Bacsal K. Spectrum of Vogt-Koyanagi-Harada disease in Singapore. Int Ophthalmol 2007;27:137-42. [Crossref] [PubMed]
- Yang P, Ye Z, Du L, et al. Novel treatment regimen of Vogt-Koyanagi-Harada disease with a reduced dose of corticosteroids combined with immunosuppressive agents. Curr Eye Res 2018;43:254-61. [Crossref] [PubMed]
- Papasavvas I, Tugal-Tutkun I, Herbort CP Jr. Vogt-Koyanagi-Harada is a Curable Autoimmune Disease: Early Diagnosis and Immediate Dual Steroidal and Non-Steroidal Immunosuppression are Crucial Prerequisites. J Curr Ophthalmol 2020;32:310-4. [Crossref] [PubMed]
- Sakata VM, da Silva FT, Hirata CE, et al. High rate of clinical recurrence in patients with Vogt-Koyanagi-Harada disease treated with early high-dose corticosteroids. Graefes Arch Clin Exp Ophthalmol 2015;253:785-90. [Crossref] [PubMed]
- Keino H, Goto H, Usui M. Sunset glow fundus in Vogt-Koyanagi-Harada disease with or without chronic ocular inflammation. Graefes Arch Clin Exp Ophthalmol 2002;240:878-82. [Crossref] [PubMed]
- Urzua CA, Herbort C Jr, Valenzuela RA, et al. Initial-onset acute and chronic recurrent stages are two distinctive courses of Vogt-Koyanagi-Harada disease. J Ophthalmic Inflamm Infect 2020;10:23. [Crossref] [PubMed]
- Ei Ei Lin Oo. Vogt-Koyanagi-Harada disease managed with immunomodulatory therapy within 3 months of disease onset. Am J Ophthalmol 2020;220:37-44. [Crossref] [PubMed]
- Paredes I, Ahmed M, Foster CS. Immunomodulatory therapy for Vogt-Koyanagi-Harada patients as first-line therapy. Ocul Immunol Inflamm 2006;14:87-90. [Crossref] [PubMed]
- Kim SJ, Yu HG. The use of low-dose azathioprine in patients with Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm 2007;15:381-7. [Crossref] [PubMed]
- Cuchacovich M, Solanes F, Díaz G, et al. Comparison of the clinical efficacy of two different immunosuppressive regimens in patients with chronic vogt-koyanagi-harada disease. Ocul Immunol Inflamm 2010;18:200-7. [Crossref] [PubMed]
- Kondo Y, Fukuda K, Suzuki K, et al. Chronic noninfectious uveitis associated with Vogt-Koyanagi-Harada disease treated with low-dose weekly systemic methotrexate. Jpn J Ophthalmol 2012;56:104-6. [Crossref] [PubMed]
- Soheilian M, Aletaha M, Yazdani S, et al. Management of pediatric Vogt-Koyanagi- Harada (VKH)-associated panuveitis. Ocul Immunol Inflamm 2006;14:91-8. [Crossref] [PubMed]
- Abu El-Asrar AM, Hemachandran S, Al-Mezaine HS, et al. The outcomes of mycophenolate mofetil therapy combined with systemic corticosteroids in acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol 2012;90:e603-8. [Crossref] [PubMed]
- Abu El-Asrar AM, Dosari M, Hemachandran S, et al. Mycophenolate mofetil combined with systemic corticosteroids prevents progression to chronic recurrent inflammation and development of 'sunset glow fundus' in initial-onset acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol 2017;95:85-90. [Crossref] [PubMed]
- Shen E, Rathinam SR, Babu M, et al. Outcomes of Vogt-Koyanagi-Harada Disease: A Subanalysis From a Randomized Clinical Trial of Antimetabolite Therapies. Am J Ophthalmol 2016;168:279-86. [Crossref] [PubMed]
- Kaçmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology 2010;117:576-84. [Crossref] [PubMed]
- Hiyama T, Harada Y, Kiuchi Y. Clinical Characteristics and Efficacy of Adalimumab and Low-Dose Methotrexate Combination Therapy in Patients With Vogt-Koyanagi-Harada Disease. Front Med (Lausanne) 2022;8:730215. [Crossref] [PubMed]
- Haruta M, Yoshioka M, Fukutomi A, et al. The Effect of Low-dose Cyclosporine (100 mg Once Daily) for Chronic Vogt-Koyanagi-Harada Disease. Nippon Ganka Gakkai Zasshi 2017;121:474-9. [PubMed]
- Jabs DA. Immunosuppression for the Uveitides. Ophthalmology 2018;125:193-202. [Crossref] [PubMed]
- Sheppard J, Joshi A, Betts KA, et al. Effect of Adalimumab on Visual Functioning in Patients With Noninfectious Intermediate Uveitis, Posterior Uveitis, and Panuveitis in the VISUAL-1 and VISUAL-2 Trials. JAMA Ophthalmol 2017;135:511-8. [Crossref] [PubMed]
- Nakai S, Takeuchi M, Usui Y, et al. Efficacy and Safety of Adalimumab for Exacerbation or Relapse of Ocular Inflammation in Patients with Vogt-Koyanagi-Harada Disease: A Multicenter Study. Ocul Immunol Inflamm 2022; Epub ahead of print. [Crossref] [PubMed]
- Yang S, Tao T, Huang Z, et al. Adalimumab in Vogt-Koyanagi-Harada Disease Refractory to Conventional Therapy. Front Med (Lausanne) 2022;8:799427. [Crossref] [PubMed]
- Jeroudi A, Angeles-Han ST, Yeh S. Efficacy of adalimumab for pediatric Vogt-Koyanagi-Harada syndrome. Ophthalmic Surg Lasers Imaging Retina 2014;45:332-4. [Crossref] [PubMed]
- Su E, Oza VS, Latkany P. A case of recalcitrant pediatric Vogt-Koyanagi-Harada disease successfully controlled with adalimumab. J Formos Med Assoc 2019;118:945-50. [Crossref] [PubMed]
- Takayama K, Obata H, Takeuchi M. Efficacy of Adalimumab for Chronic Vogt-Koyanagi-Harada Disease Refractory to Conventional Corticosteroids and Immunosuppressive Therapy and Complicated by Central Serous Chorioretinopathy. Ocul Immunol Inflamm 2020;28:509-12. [Crossref] [PubMed]
- Niccoli L, Nannini C, Cassarà E, et al. Efficacy of infliximab therapy in two patients with refractory Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2009;93:1553-4. [Crossref] [PubMed]
- Wang Y, Gaudio PA. Infliximab therapy for 2 patients with Vogt-Koyanagi-Harada syndrome. Ocul Immunol Inflamm 2008;16:167-71. [Crossref] [PubMed]
- Zmuda M, Tiev KP, Knoeri J, et al. Successful use of infliximab therapy in sight-threatening corticosteroid-resistant Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm 2013;21:310-6. [Crossref] [PubMed]
- Budmann GA, Franco LG, Pringe A. Long term treatment with infliximab in pediatric Vogt-Koyanagi-Harada disease. Am J Ophthalmol Case Rep 2018;11:139-41. [Crossref] [PubMed]
- Khalifa YM, Bailony MR, Acharya NR. Treatment of pediatric vogt-koyanagi-harada syndrome with infliximab. Ocul Immunol Inflamm 2010;18:218-22. [Crossref] [PubMed]
- Abu El-Asrar AM, Dheyab A, Khatib D, et al. Efficacy of B Cell Depletion Therapy with Rituximab in Refractory Chronic Recurrent Uveitis Associated with Vogt-Koyanagi-Harada Disease. Ocul Immunol Inflamm 2022;30:750-7. [Crossref] [PubMed]
- Bolletta E, Gozzi F, Mastrofilippo V, et al. Efficacy of Rituximab Treatment in Vogt-Koyanagi-Harada Disease Poorly Controlled by Traditional Immunosuppressive Treatment. Ocul Immunol Inflamm 2022;30:1303-8. [Crossref] [PubMed]
- Umran RMR, Shukur ZYH. Rituximab for sight-threatening refractory pediatric Vogt-Koyanagi-Harada disease. Mod Rheumatol 2018;28:197-9. [Crossref] [PubMed]
- Caso F, Rigante D, Vitale A, et al. Long-lasting uveitis remission and hearing loss recovery after rituximab in Vogt-Koyanagi-Harada disease. Clin Rheumatol 2015;34:1817-20. [Crossref] [PubMed]
Cite this article as: Choo CH, Acharya NR, Shantha JG. Comprehensive and updated review on the diagnosis and treatment of Vogt-Koyanagi-Harada disease. Ann Eye Sci 2023;8:4.


