
Comparison of ocular biometry in Chinese patients using two swept-source optical coherence tomography devices
Highlight box
Key findings
• Comparisons showed significant differences in most parameters measured using the two devices (ANTERION vs. IOLmaster 700), but the differences were so small that they are not clinically relevant except for the TK and WTW.
What was known and what is new?
• SS-OCT provides reliable ocular biometry measurements. Only one other study has compared ocular measurements between the two studied devices in Chinese patients but did not include the TK measurement. Our study looked at a different cohort of Chinese patients, adding to the available evidence and we also included TK measurements.
What is the implication, and what should change now?
• The two devices do not show a clinically relevant difference when used for measurement of AL, IOL power, CCT and LT. However, TK and WTW measurements should not be used interchangeably between the two devices.
Introduction
Background
Accurate optical biometry measurement is critically important in the preparation of cataract surgery as it helps to achieve the best postoperative refractive outcomes for patients. Partial coherence interferometry (PCI) device such as IOLMaster 500 (Carl Zeiss Meditec AG, Jena, Germany) have been the gold standard for optical biometry. However, these devices have difficulties in measuring dense cataracts or severe posterior subcapsular (PSC) cataract due to the use of a shorter wavelength (780 nm) laser diode infrared light (1). They also could not provide data on the posterior corneal surface, which gives a more accurate measurement for those with an altered cornea (2), e.g., previous myopic laser refractive surgery or keratoconus eyes. The posterior corneal surface measurement also brings accuracy of measurement to those having toric intraocular lens (IOL) implantation (3). It is expected that more patients who had previously undergone refractive surgery, will undergo cataract surgery. Therefore, it will become more important to accurately measure total corneal power (2).
The IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) is the first swept-source optical coherence tomography (SS-OCT) biometric device. It uses a longer wavelength light source, which leads to an increase in the success rate of axial length (AL) measurement compared with PCI devices (4-6), especially in measuring AL in longer eyes (AL ≥30.0 mm) (7). Another SS-OCT device, ANTERION (Heidelberg Engineering, Germany), uses a 1,300 nm central wavelength of light. It provides a scan depth range of 32 mm for the AL and an in-tissue axial resolution of <10 µm. It uses OCT-based structural images to generate ocular biometric measurements (8).
Rationale and knowledge gap
The biometry measurements between IOLMaster and ANTERION have previously been evaluated in cataract patients with good agreement, however few parameters such as the anterior keratometry (K), anterior chamber depth (ACD), lens thickness (LT) and white-to-white (WTW) were shown to have discrepancies and advised not to be used interchangeably (8-13). So far, only one study has compared ocular measurement between the two devices in Chinese patients, however the authors did not compare the total keratometry (TK) measurement, and supported only AL measurement to be interchangeable between the two devices (13).
Objective
The purpose of this study was to report the level of agreement of all ocular biometric measurements between the IOLMaster 700 and ANTERION biometer in a cohort of Chinese patients.
Reporting guideline
We present this article in accordance with the GRRAS reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-22-77/rc).
Methods
This retrospective study comprised of cataract patients from the Department of Ophthalmology, United Christian Hospital (UCH), Hong Kong. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Hong Kong Kowloon East Research Ethics Committee (No. KE-21-0260/ER-3) and individual consent for this retrospective analysis was waived.
All patients who underwent routine cataract surgery between March and July 2021 had sequential biometry with both SS-OCT devices on the same day under mesopic conditions prior to pupil dilation. Patients who had a history of ocular trauma, other ocular surgeries, corneal or other ocular diseases that could affect outcomes, or previous contact lens wear; or patients who were unable to cooperate or fixate adequately were excluded. The following biometry parameters were compared: K, TK, AL, central corneal thickness (CCT), ACD (ACD minus CCT for IOLMaster 700), LT, WTW and IOL power. The IOL power for emmetropia was evaluated with an A-constant of 119.3 (TECNIS, Johnson & Johnson Vision). All cataracts were classified and graded into three groups: cortical opacity (CO), nuclear opacity (NO), and PSC. The severity of each item was graded from 1 (early cataract) to 6 (mature cataract) for the NO and from 1 to 5 for the cortical and PSC opacities according to the Lens Opacities Classification System (LOS) III grading system (14).
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 24.0. For normally distributed data, continuous variables were presented in mean & standard deviation (SD). Otherwise, data would be presented in median & interquartile range (IQR). Each parameter between the two biometers was compared with Wilcoxon signed-rank test (15). Results were defined as statistically significant if P value was less than 0.05. To assess the agreement between the devices, Bland-Altman analysis with 95% limits of agreement (LoA) was used, as it allows identification of any systematic difference between the measurements or possible outliers (16). The results derived from right eyes and left eyes were reported separately, to avoid any difference in results that may be due to biased statistical analysis when using both eyes together, as previously reported (17).
Results
In total, 92 eyes of 47 patients with cataract (24 males, 23 females) were included in the study. Their mean age was 68.7 (range, 44–84) years. Table 1 shows the descriptive summary with the median and IQR of each parameter. When using the right eyes in the analysis, all parameters apart from the flat K, AL and IOL power were statistically different between the two biometers. When using only the left eyes for the analysis, all parameters apart from the flat K and the steep K showed statistically significant differences between measurements taken from the two biometers. Table 2 shows the mean differences and LoA for all parameters. Figures 1-3 show the Bland-Altman plots for all parameters measured in both eyes, right and left eyes respectively.
Table 1
| Parameters | Right eye | Left eye | |||
|---|---|---|---|---|---|
| Median (IQR) | P valuea | Median (IQR) | P valuea | ||
| K flat (D) | 0.815 | 0.307 | |||
| IOLM | 43.36 (2.63) | 43.5 (2.19) | |||
| ANTERION | 43.22 (2.67) | 43.38 (2.48) | |||
| K steep (D) | 0.002* | 0.064 | |||
| IOLM | 44.31 (2.68) | 44.32 (2.37) | |||
| ANTERION | 44.04 (2.74) | 44.26 (2.62) | |||
| TK flat (D) | 0.000* | 0.000* | |||
| IOLM | 43.29 (2.58) | 43.44 (2.30) | |||
| ANTERION | 42.56 (2.65) | 42.71 (2.52) | |||
| TK steep (D) | 0.000* | 0.000* | |||
| IOLM | 44.31 (2.78) | 44.32 (2.52) | |||
| ANTERION | 43.55 (3.03) | 43.91 (2.44) | |||
| AL (mm) | 0.220 | 0.013* | |||
| IOLM | 23.73 (1.90) | 23.65 (1.79) | |||
| ANTERION | 23.64 (1.94) | 23.64 (1.79) | |||
| CCT (μm) | 0.000* | 0.000* | |||
| IOLM | 549 (26.5) | 549 (28.0) | |||
| ANTERION | 545 (27.0) | 545 (27.0) | |||
| ACD (mm) | 0.000* | 0.000* | |||
| IOLM | 2.39 (0.62) | 2.45 (0.67) | |||
| ANTERION | 2.48 (0.63) | 2.49 (0.62) | |||
| LT (mm) | 0.000* | 0.000* | |||
| IOLM | 4.74 (0.45) | 4.69 (0.44) | |||
| ANTERION | 4.80 (0.45) | 4.78 (0.48) | |||
| WTW (mm) | 0.002* | 0.000* | |||
| IOLM | 11.90 (0.70) | 12.0 (0.50) | |||
| ANTERION | 11.74 (0.64) | 11.72 (0.69) | |||
| IOL power (D) | 0.058 | 0.039* | |||
| IOLM | 20.5 (4.0) | 20.5 (4.13) | |||
| ANTERION | 20.5 (3.75) | 20.5 (4.0) | |||
a, assessed differences by Wilcoxon signed rank test (statistically significant results in *). K, keratometry; TK, total keratometry; AL, axial length; CCT, central corneal thickness; ACD, anterior chamber depth; LT, lens thickness; WTW, white-to-white; IOL, intraocular lens; D, Diopter; IQR, interquartile range.
Table 2
| Parameters | Both eyes | Right eyes | Left eyes | |||||
|---|---|---|---|---|---|---|---|---|
| Differencea | LoAb | Differencea | LoAb | Differencea | LoAb | |||
| K flat (D) | 0.034±0.287 | −0.53, 0.60 | −0.007±0.18 | −0.37, 0.35 | 0.04±0.22 | −0.39, 0.47 | ||
| K steep (D) | 0.10± 0.232 | −0.35, 0.56 | 0.06±0.17 | −0.28, 0.40 | 0.04±0.17 | −0.29, 0.38 | ||
| TK flat (D) | 0.56±0.308 | −0.04, 1.164 | 0.26±0.35 | −0.43,0.96 | 0.30±0.36 | −0.41, 1.0 | ||
| TK steep (D) | 0.58±0.248 | 0.09, 1.066 | 0.29±0.35 | −0.39, 0.97 | 0.29±0.33 | −0.37, 094 | ||
| AL (mm) | −0.006±0.105 | −0.211, 0.20 | −0.009±0.10 | −0.21, 0.20 | 0.003±0.01 | −0.02, 0.026 | ||
| CCT (μm) | 4.61±3.888 | −3.01, 12.23 | 1.98±3.4 | −4.69, 8.64 | 2.63±3.75 | −4.72, 9.98 | ||
| ACD (mm) | −0.07±0.016 | −0.105, -0.04 | −0.04±0.04 | −0.13, 0.04 | −0.03±0.03 | −0.10, 0.03 | ||
| LT (mm) | −0.07±0.094 | −0.25, 0.118 | −0.03±0.08 | −0.19, 0.13 | −0.04±0.065 | −0.17, 0.088 | ||
| WTW (mm) | 0.21±0.392 | −0.56, 0.97 | 0.08±0.29 | −0.48, 0.65 | 0.122±0.30 | −0.47, 0.71 | ||
| IOL power (D) | −0.09±0.286 | −0.65, 0.472 | −0.07±0.25 | −0.57, 0.43 | −0.11±0.31 | −0.73, 0.51 | ||
a, differences between two devices were defined by subtracting the latter (ANTERION) mean value from the former (IOLMaster) mean value ± standard deviation; b, Bland-Altman analysis with 95% LoA. K, keratometry; D, Diopter; TK, total keratometry; AL, axial length; mm, millimeters; CCT, central corneal thickness; μm, micrometers; ACD, anterior chamber depth; LT, lens thickness; WTW, white-to-white; IOL, intraocular lens; LoA, limits of agreement.
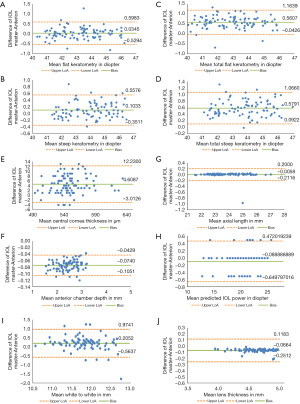
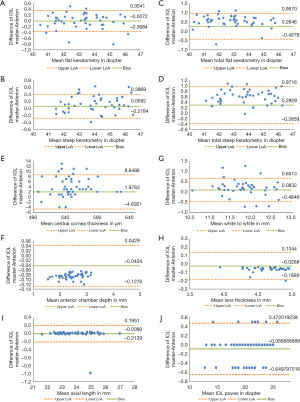

IOLMaster did not obtain WTW in 2 cases (2.17%). The ANTERION did not obtain AL in 1 case (1.09%). The lens grade of this eye was NO2 according to the LOS III (14); and the AL measured by IOLMaster for this eye was 25.13 mm. The ANTERION also did not obtain ACD in 2 cases (2.17%) and LT in 5 cases (5.43%). The lens grade of eyes with missing ACD were both PSC1; and the lens grades of those with missing LT were NO1, NO1, NO2, NO3 and NO3.
Discussion
Comparison with similar research
Several studies have compared the biometry measurement between IOLMaster 700 and ANTERION (8-13). Although most reported a good agreement, they all showed discrepancies in several parameters and advised against some parameters to be used interchangeably. To the best of our knowledge, there was only one other study that compared ocular measurements between the two studied devices in Chinese patients (13). The authors reported all parameters were not interchangeable apart from AL, and they did not include the TK values (13). Our study looked at a different cohort of Chinese patients, adding to the available evidence and included TK measurements.
When comparing IOLMaster 700 with another SS-OCT biometer such as the Argos (Movu, Inc.), Sabatino et al. found significant differences in most parameters apart from AL (18). Despite a very high positive correlation and high agreement were found for AL, mean K, ACD, LT and CCT measurements, the authors advised that both ACD and corneal diameters should not be used interchangeably between these two devices. Oh et al. compared biometry measurements with three different SS-OCT biometers namely IOLMaster 700, ANTERION and CASIA2 and found that the TK values of each device were different and advised it not to be used interchangeably when calculating IOLs (10).
Key findings and explanation of findings
In our study, there were significant differences in AL when the left eyes were used for the analysis, however not when the right eyes were used for the analysis. For the left eyes analysis, the mean difference was 0.003 mm (P=0.013), which is in concordance to previous studies (8-13). Given that an AL measurement error of 1 mm induces 2.5 D deviation in IOL calculation in the eye with an average AL of 23.5 mm (11), this AL difference would result in 0.02 D of IOL power difference. The AL measurements between two devices are therefore of clinical insignificance and can be considered interchangeable.
The flat K measurements showed no statistical differences between the devices however the steep K showed significant differences in the right eyes. The mean difference of ≤0.1 D is, similar to those reported by others, of no clinical relevance (8,9). Like others (8,11,13), IOLMaster in our study was found to produce a slightly steeper K values compared with those of ANTERION. It was suggested that such findings could be attributed to the different technologies and measurement zones used in each device (8). Some reported that the higher the keratometric value, the greater the difference between the two devices, resulting in their steep K value using IOLMaster of up to 0.8 D difference, and advised against the use of keratometric values from these devices interchangeably in IOL calculation or keratoconus (KC) follow-up (11). We did not find such results in our study, and we did not have keratoconus patients in our cohort.
For the CCT value, ANTERION measured slightly thinner values compared to IOLMaster, similar to findings in other studies (8,13). Fişuş et al. suggested that such difference could be explained by the different use of refractive index between the devices (8). Our mean difference was <5 µm which was statistically significant when using right eyes or left eyes, but might not be clinically relevant as it is not commonly included in IOL power calculation. It could however be essential to glaucoma screening, in preoperative assessment for refractive surgery or monitoring corneal ectasia. In that case we suggest the use of other non-contact device such as those with spectral-domain optical coherence tomographer or Scheimpflug-Placido topographer for the evaluation and follow up of CCT.
Our LT measurements from ANTERION was slightly thicker by significant mean differences of <0.07 mm, consistent with those previously reported by others (8,9,11,12). IOL formulas such as Olsen and Holladay use LT for IOL power calculation (19,20). As reported by Sabatino et al., an increase of 0.2 mm in LT would affect the IOL power by 0.2 D (18). Hence, a difference of <0.07 mm might not be clinically relevant.
We found a higher value in ACD measured with ANTERION compared to IOLMaster, when using right eyes or left eyes for the analysis. As graphically represented in the Bland Altman plots Figures 1-3, it can be seen that ACD consistently measured higher with ANTERION than with IOLMaster by a mean of <0.07 mm. This difference may not be clinically relevant in IOL calculation as it was reported that a change in ACD of 0.07 mm corresponds to a <0.08 D change in predicted IOL power (21). It may however affect the pre- and post-operative assessment in phakic IOL (pIOL) implantation. ANTERION measures anterior aqueous depth whereas IOLMaster measures ACD from the corneal epithelium to the anterior lens surface. These findings are in concordance with the study by Fişuş et al. who do not consider the ACD between these two devices to be interchangeable (8).
Our WTW measurement showed a significant mean difference of <0.2 mm when using right eyes or left eyes for the analysis, with higher value measured by IOLMaster, similar to other studies (9,11,12). WTW is a variable used in IOL power calculation formulas and the implantation of pIOLs such as the implantable collamer lens (ICL) for the correction of refractive errors. Considering that ICLs are sized to the nearest 0.50 mm, it has been suggested that a difference ≥0.50 mm in the WTW distance is clinically significant (22). Although our mean difference is small, the LoA was wide (ranging from −0.56 to 0.97). It was reported that a change in WTW of 0.2 mm would affect the prediction error by <0.1 D (21). Hence the WTW measurements between the two biometers should not be used interchangeably.
We found a significant mean difference of >0.5 D in TK measurements, with a wider LoA than that of the anterior K measurements, similar to that found by Oh et al. (10). The authors commented such discrepancy could be due to the difference in the algorism used between the devices. IOLMaster 700 first builds a toric anterior surface model from the telecentric 3-zone K and then measures pachymetry using SS-OCT in the six meridians. The pachymetry values are fitted to the anterior surface model to create the toric posterior surface model. The TK is then calculated from the anterior and posterior corneal curvatures (23). Whereas ANTERION uses SS-OCT technology to acquire 65 radial scans and generate all data, including K data, in 4 zones (2, 4, 6, and 8 mm) and within a 3-mm ring (8). We would therefore advise against using the TK values from these two devices interchangeably when calculating IOL power especially for toric IOL selection. The measurement from IOLMaster 700 would be preferred in this case due to its ability to achieve good refractive outcome (2).
In the IOL predicted power, we found the mean differences of 0.07 D in the right eyes and 0.11 D in the left eyes to be statistically significant but clinically irrelevant. Pfaeffli et al. showed that in eyes with normal AL, there was a high agreement between the two biometers regarding the predicted refraction and postoperative outcome among the seven IOL power formulas investigated (12). Though they also reported that for modern formulas which involve ACD and LT, there was worse agreement in IOL power calculation compared to formulas that do not involve these parameters, and suggested these two parameters should not be used interchangeably from the two devices.
In our study, the ANTERION did not obtain ACD, AL and LT in 2 (2.17%), 1 (1.09%) and 5 cases (5.43%) respectively, and their cataract status was not dense. ANTERION has a higher wavelength than IOLMaster 700 (1,300 vs. 1,050 nm), resulting in a higher AL acquisition rate because longer wavelengths improve penetration (1). However like others (10,11), we had AL measurement failure in one patient with ANTERION. The cataract in this case was moderately dense with an AL of 25 mm. It was commented that the different acquisition method could be the cause of this discrepancy (10,11); with IOLMaster 700 measuring AL by the average values of 3 scans in each of the six meridians (24), whereas ANTERION obtains AL by averaging 3 consecutive 3 subsets of data (11).
Strengths and limitations
This study has several limitations. First, this is a retrospective study, which affected the data available for analysis resulting in a relatively small sample size, in turn may affect the LoA interval and over-estimate the true differences. Second, all included patients had cataract; hence the results of this study cannot be extrapolated to patients with corneal pathologies, for example, KC or previous refractive surgeries. Further investigation in a prospective study design, including both normal and diseased eyes as well as comparing the postoperative refractive outcomes data in cataract patients to evaluate the IOL power accuracy, will provide more information about the agreements between these devices. Third, all patients were of Chinese origin. Therefore, our data may not be generalizable to other ethnicities.
Implications and actions needed
The implications of this study are that the two biometers ANTERION and IOL master 700 have good agreement in most parameters when measuring on Chinese patients. When using the two biometers interchangeably, the differences are clinically irrelevant, except for when measuring the TK and the WTW. The TK and WTW therefore should not be used interchangeably between the two machines.
Conclusions
In summary, the two biometers showed good agreement in most parameters with clinically acceptable LoA. Comparisons showed a small but significant difference in most parameters that are not clinically relevant, except for TK and WTW. These two parameters measured from the two biometers should not be used interchangeably.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the GRRAS reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-22-77/rc
Data Sharing Statement: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-77/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-77/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hong Kong Kowloon East Research Ethics Committee (No. KE-21-0260/ER-3) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirnschall N, Varsits R, Doeller B, et al. Enhanced Penetration for Axial Length Measurement of Eyes with Dense Cataracts Using Swept Source Optical Coherence Tomography: A Consecutive Observational Study. Ophthalmol Ther 2018;7:119-24. [Crossref] [PubMed]
- Lawless M, Jiang JY, Hodge C, et al. Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery. Clin Exp Ophthalmol 2020;48:749-56. [Crossref] [PubMed]
- Koch DD, Ali SF, Weikert MP, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 2012;38:2080-7. [Crossref] [PubMed]
- Yang CM, Lim DH, Kim HJ, et al. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS One 2019;14:e0223114. [Crossref] [PubMed]
- Chan TCY, Yu MCY, Chiu V, et al. Comparison of two novel swept-source optical coherence tomography devices to a partial coherence interferometry-based biometer. Sci Rep 2021;11:14853. [Crossref] [PubMed]
- McAlinden C, Wang Q, Gao R, et al. Axial Length Measurement Failure Rates With Biometers Using Swept-Source Optical Coherence Tomography Compared to Partial-Coherence Interferometry and Optical Low-Coherence Interferometry. Am J Ophthalmol 2017;173:64-9. [Crossref] [PubMed]
- Tan Y, Liu L, Li J, et al. Evaluation of preoperative corneal astigmatism using swept-source optical biometry in Chinese cataract surgery candidates with high myopia: a prospective, comparative observational study. Ann Transl Med 2021;9:618. [Crossref] [PubMed]
- Fişuş AD, Hirnschall ND, Findl O. Comparison of 2 swept-source optical coherence tomography-based biometry devices. J Cataract Refract Surg 2021;47:87-92. [Crossref] [PubMed]
- Shetty N, Kaweri L, Koshy A, et al. Repeatability of biometry measured by three devices and its impact on predicted intraocular lens power. J Cataract Refract Surg 2021;47:585-92. [Crossref] [PubMed]
- Oh R, Oh JY, Choi HJ, et al. Comparison of ocular biometric measurements in patients with cataract using three swept-source optical coherence tomography devices. BMC Ophthalmol 2021;21:62. [Crossref] [PubMed]
- Panthier C, Rouger H, Gozlan Y, et al. Comparative analysis of 2 biometers using swept-source OCT technology. J Cataract Refract Surg 2022;48:26-31. [Crossref] [PubMed]
- Pfaeffli OA, Weber A, Hoffer KJ, et al. Agreement of intraocular lens power calculation between 2 SS-OCT-based biometers. J Cataract Refract Surg 2022;48:535-41. [Crossref] [PubMed]
- Dong J, Yao J, Chang S, et al. Comparison Study of the Two Biometers Based on Swept-Source Optical Coherence Tomography Technology. Diagnostics (Basel) 2022;12:598. [Crossref] [PubMed]
- Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831-6. [Crossref] [PubMed]
- Fay MP, Proschan MA. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat Surv 2010;4:1-39. [Crossref] [PubMed]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135-60. [Crossref] [PubMed]
- Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt 2013;33:7-14. [Crossref] [PubMed]
- Sabatino F, Matarazzo F, Findl O, et al. Comparative analysis of 2 swept-source optical coherence tomography biometers. J Cataract Refract Surg 2019;45:1124-9. [Crossref] [PubMed]
- Olsen T, Hoffmann P. C constant: new concept for ray tracing-assisted intraocular lens power calculation. J Cataract Refract Surg 2014;40:764-73. [Crossref] [PubMed]
- Hoffer KJ. Clinical results using the Holladay 2 intraocular lens power formula. J Cataract Refract Surg 2000;26:1233-7. [Crossref] [PubMed]
- Teshigawara T, Meguro A, Mizuki N. Influence of pupil dilation on the Barrett universal II (new generation), Haigis (4th generation), and SRK/T (3rd generation) intraocular lens calculation formulas: a retrospective study. BMC Ophthalmol 2020;20:299. [Crossref] [PubMed]
- Sanders DR, Vukich JA, Doney K, et al. U.S. Food and Drug Administration clinical trial of the Implantable Contact Lens for moderate to high myopia. Ophthalmology 2003;110:255-66. [Crossref] [PubMed]
- Savini G, Taroni L, Schiano-Lomoriello D, et al. Repeatability of total Keratometry and standard Keratometry by the IOLMaster 700 and comparison to total corneal astigmatism by Scheimpflug imaging. Eye (Lond) 2021;35:307-15. [Crossref] [PubMed]
- Akman A, Asena L, Güngör SG. Evaluation and comparison of the new swept source OCT-based IOLMaster 700 with the IOLMaster 500. Br J Ophthalmol 2016;100:1201-5. [Crossref] [PubMed]
Cite this article as: Ho VW, Nguyen HC, Wong SH, Li AL, Li KKW. Comparison of ocular biometry in Chinese patients using two swept-source optical coherence tomography devices. Ann Eye Sci 2023;8:9.


